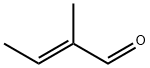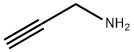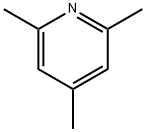
2,4,6-Collidine synthesis
- Product Name:2,4,6-Collidine
- CAS Number:108-75-8
- Molecular formula:C8H11N
- Molecular Weight:121.18
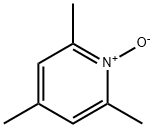
3376-50-9
14 suppliers
inquiry

108-75-8
432 suppliers
$5.00/10g
Yield:108-75-8 90%
Reaction Conditions:
with bis(triphenyl)oxodiphosphonium trifluoromethanesulfonate salt;potassium iodide in ethanol at 20; for 1.08333 h;
Steps:
Typical procedure for the deoxygenationof pyridine-N-oxide
General procedure: To a 25-mL flask containing a stirred solution of pyridine-N-oxide (1 mmol, 0.095 g) in EtOH (3 mL), Hendrickson reagent (1 mmol, 0.839 g), and KI (2 mmol, 0.336 g) were added. The mixture was stirred magnetically at room temperature, and monitored by TLC. Upon completion of the reaction, the solvent was evaporated, and then saturated sodium bicarbonate solution (10 mL) was added. The product was extracted with chloroform (3 × 5 mL), and dried over anhydrous MgSO4. The filtrate was evaporated, and pyridine (0.059 g, 75%) was obtained as the sole product.
References:
Khodaei, Mohammad Mehdi;Alizadeh, Abdolhamid;Hezarkhani, Hadis Afshar [Journal of the Iranian Chemical Society,2018,vol. 15,# 8,p. 1843 - 1849]

67-64-1
6 suppliers
$19.10/10ml

108-75-8
432 suppliers
$5.00/10g

67-64-1
6 suppliers
$19.10/10ml

108-75-8
432 suppliers
$5.00/10g
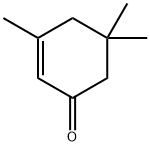
78-59-1
548 suppliers
$11.00/25g
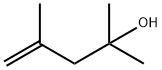
19781-53-4
22 suppliers
$518.00/100g

67-64-1
6 suppliers
$19.10/10ml

108-75-8
432 suppliers
$5.00/10g

78-59-1
548 suppliers
$11.00/25g

19781-53-4
22 suppliers
$518.00/100g
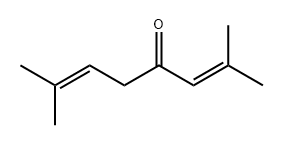
63426-15-3
0 suppliers
inquiry