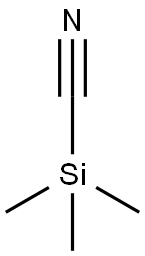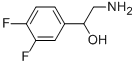
2-amino-1-(3,4-difluorophenyl)ethanol synthesis
- Product Name:2-amino-1-(3,4-difluorophenyl)ethanol
- CAS Number:10145-04-7
- Molecular formula:C8H9F2NO
- Molecular Weight:173.161
Yield:-
Reaction Conditions:
Stage #1: trimethylsilyl cyanide;3,4 difluorobenzaldehydewith zinc(II) iodide in tetrahydrofuran at 0; for 3.5 h;Inert atmosphere;
Stage #2: with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20; for 21 h;Inert atmosphere;
Stage #3: with water;sodium hydroxide in tetrahydrofuran at 0; for 1 h;Inert atmosphere;
Steps:
130.A
Step A: 2-Amino-1-(3.4-difluoro-phenyl)-ethanol. To a solution consisting of 3,4-difluorobenzaldehyde (3.85 g, 27.1 mmol), zinc iodide (104.9 mg, 0.329 mmol) and THF (5 mL) at 0 °C under nitrogen was added TMSCN (4.60 mL, 33.9 mmol). The resultant mixture was stirred for 3.5 h at 0 °C and then cannulated into a 0 °C suspension of LiAIH4 (1.41 g, 67.7 mmol) in THF (80 mL). The resultant mixture was allowed to gradually warm to rt with stirring for 21 h. The reaction mixture was then re-cooled to 0°C and carefully treated with water (2.57 mL) followed by 15% aq. NaOH (2.57 mL) and lastly with water (7.7 mL). The resultant mixture was stirred for 1 h, the solids removed by vacuum filtration and the filtrate concentrated. The resulting crude product was purified by FCC (CH2Cl2ZMeOHZNH3) to give the desired product.
References:
WO2009/105220,2009,A1 Location in patent:Page/Page column 96
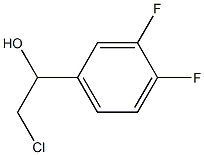
51336-97-1
21 suppliers
inquiry

10145-04-7
11 suppliers
inquiry
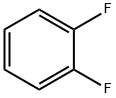
367-11-3
305 suppliers
$10.00/1g

10145-04-7
11 suppliers
inquiry
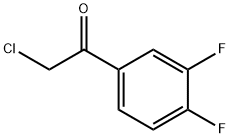
51336-95-9
238 suppliers
$15.00/1g

10145-04-7
11 suppliers
inquiry
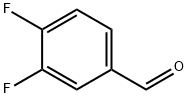
34036-07-2
355 suppliers
$7.00/5g

10145-04-7
11 suppliers
inquiry