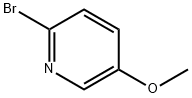
2-BROMO-5-METHOXYPYRIDINE synthesis
- Product Name:2-BROMO-5-METHOXYPYRIDINE
- CAS Number:105170-27-2
- Molecular formula:C6H6BrNO
- Molecular Weight:188.02
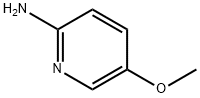
10167-97-2
223 suppliers
$18.00/1g

105170-27-2
184 suppliers
$8.00/1g
Yield:105170-27-2 63%
Reaction Conditions:
with sodium hydroxide;bromine;sodium nitrite in water;hydrogen bromide
Steps:
36.a L-3,5-Diiodo-3'-(5-hydroxy-2-pyridylmethyl)-thyronine
(a) 2-Amino-5-methoxypyridine (14.8 g, prepared by the method of J.G. Lombardino, J. Med. Chem., 1981, 24, 39) was dissolved in 60% hydrobromic acid (150 ml) and to the cooled (-10°) stirred solution bromine (47.47 g) was added dropwise. To the resulting yellow suspension was added, dropwise, sodium nitrite (20.53 g) in water (40 ml), keeping the temperature below -5°. The mixture was stirred to room temperature, and after 0.5 hour cooled to 0°, and a solution of sodium hydroxide (120 g) in water (100 ml) was slowly added. The mixture was thoroughly extracted with ether, the combined ether extracts dried with anhydrous sodium sulphate, and evaporated. The residue was chromatographed on silica gel (150 g). Elution with dichloromethane gave a yellow oil (14.1 g, 63%) which was combined with a smaller batch (3.4 g) and distilled under reduced pressure to give 2-bromo-5-methoxypyridine (16.4 g). b.p. 76°-78°/0.6 torr.
References:
Smith Kline & French Laboratories Ltd. US4766121, 1988, A
55717-45-8
279 suppliers
$5.00/250mg

74-88-4
339 suppliers
$15.00/10g

105170-27-2
184 suppliers
$8.00/1g
624-28-2
713 suppliers
$5.00/5g

105170-27-2
184 suppliers
$8.00/1g
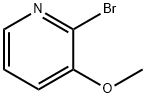
24100-18-3
251 suppliers
$6.00/1g

105170-27-2
184 suppliers
$8.00/1g
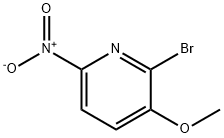
76066-07-4
92 suppliers
$44.00/250mg

105170-27-2
184 suppliers
$8.00/1g