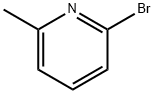
2-Bromo-6-methylpyridine synthesis
- Product Name:2-Bromo-6-methylpyridine
- CAS Number:5315-25-3
- Molecular formula:C6H6BrN
- Molecular Weight:172.02
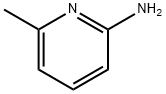
1824-81-3
472 suppliers
$8.00/25g

5315-25-3
454 suppliers
$6.00/5g
Yield:5315-25-3 95%
Reaction Conditions:
Stage #1: 2-Amino-6-methylpyridinewith hydrogen bromide;bromine at -10 - -5; for 1.5 h;
Stage #2: with NaNO2 in water monomer at -10 - -5; for 1.5 h;
Stage #3: with sodium hydroxide in water monomer at -10;
Steps:
4.2.1. Synthesis of 2-bromo-6-methylpyridine (2)
31 g (286.6 mmol) of 2-aminopicolin was taken in a 1lt roundbottom flask, to this 170 mL of 48% HBr was added at room temperature.Then the mixture was cooled in a freezing mixture of about -10 °C. Then 40 mL of Br2 was added dropwise over a periodof 40 min with stirring, the orange color mixture was again stirredat -5 °C for 1.5 h. Then 55 g (779.2 mmol) of NaNO2 was dissolved in70 mL of H2O and was added dropwise at the same temperatureand stirred for a period of another 1.5 h. Then 200 g (5 mol) of NaOHwas dissolved in 200 mL of H2O cooled in a ice-bath and addeddropwise to the reaction mixture at -10 °C with stirring. Careshould be taken that the temperature inside the flash must not rise above 0 °C, and was allowed to come to the room temperatureslowly. Then the mixture was extracted with 200 mL (25x8) ofdiethyl ether in fractions, all fractions are combined and shaken with anhydrous MgSO4, filtered and evaporated in rotary to give45 g of brown oil (95% yield).
References:
Baral, Minati;Dash, Dibyajit;Kanungo, B. K. [Journal of Molecular Structure,2020,vol. 1222]
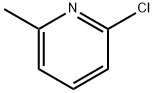
18368-63-3
361 suppliers
$5.00/1g

5315-25-3
454 suppliers
$6.00/5g
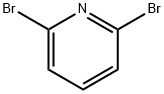
626-05-1
497 suppliers
$9.00/10g
917-54-4
168 suppliers
$52.10/25ml

5315-25-3
454 suppliers
$6.00/5g

