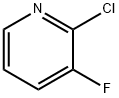
2-Chloro-3-fluoropyridine synthesis
- Product Name:2-Chloro-3-fluoropyridine
- CAS Number:17282-04-1
- Molecular formula:C5H3ClFN
- Molecular Weight:131.54
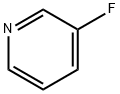
372-47-4
365 suppliers
$5.00/1g

17282-04-1
358 suppliers
$7.00/10g
Yield:17282-04-1 63%
Reaction Conditions:
Stage #1:3-Fluoropyridine with 1,4-diaza-bicyclo[2.2.2]octane;n-butyllithium in diethyl ether;hexane at -65; for 1.16667 h;
Stage #2: with hexachloroethane in diethyl ether;hexane at -40; for 2.25 h;
Steps:
1 EXAMPLE [1] [8-FLUORO-3- [3-(PYRIDIN-3-VL) HENYLLIMIDAZO [L, 2-AJPSRIDINE]
n-Butyllithium (2.5 M in hexanes; 230 ml, 0.57 mol) was added to a solution of 1, [4-DIAZABICYCLO] [[2.] 2. [2]] octane (pre-dried by azeotropic removal of water with toluene) (63. 8 g, 0.57 mol) in [ET20] (2. [5 1)] holding the temperature between-20 [AND-30°C.] After stirring for 1 h, the temperature was adjusted to-65°C and a solution of [3-FLUOROPYRIDINE] (50.0 g, 0.52 mol) in [ET20] (250 ml) was added dropwise over 10 min and the mixture stirred for a further 1 h. A solution of hexachloroethane (136.5 g, 0.58 mol) in [ET20] (350 ml) was then added dropwise over 15 min, holding the temperature [BELOW-60°C] and the mixture was stirred for 2 h, allowing the temperature to rise [TO-40°C.] The reaction was quenched by addition of saturated aqueous [NH4C1] solution (250 ml), warmed to ambient temperature and separated. The aqueous phase was extracted with [ET20] (2 x 250 ml) and the combined organics washed with further saturated aq. [NH4C1] (250 [ML),] dried over anhydrous [MGS04] and concentrated in vacuo. The residue was diluted with isohexane (150 ml) and washed with 2 N hydrochloric acid (3 x 100 ml), followed by 37% hydrochloric acid (3 x 50 ml). The acid washings were then extracted with isohexane (3 x 100 ml), basified to pH 14 by careful addition of 4 N aqueous [NAOH] solution (500 ml) and re-extracted with [CH2CL2] (3 x 150 ml). The organic fractions were dried over anhydrous [MGS04] and concentrated in vacuo. The residue was purified by distillation (bp [74-82°C,] 1 atm) to afford 2-chloro-3- fluoropyridine as a straw-coloured oil (42.6 g, 63%): [8H] (400 MHz, CDC13) 7.25-7. 30 [(1H,] m), 7.47-7. 51 [(1H,] m), 8.24 [(1H,] dd, [J 0.] 8 and 4.7) ; m/z (ES+) 132 (100%, [[MH] +).] A mixture of 2-chloro-3-fluoropyridine (21.0 g, 0.16 mol), benzophenone imine (32.3 g, 0.18 mol), [CS2CO3] (73.0 g, 0.23 mol), BINAP (5.96 g, 9.6 mmol), palladium (II) acetate (1.45 g, 6.4 mmol) and toluene (370 ml) were heated to [95°C] for 42 h, then cooled, filtered and the residue extracted with further toluene (2 x 210 ml). The filtrate was washed with 0.5 N hydrochloric acid (200 ml) and saturated aqueous [NAHCO3] (200 ml), dried over anhydrous [MGS04] and concentrated in vacuo, affording crude benzhydrylidene [(3-FLUOROPYRIDIN-2-YL) AMINE] as a brown oil (49.4 g) which was used directly without further purification: [N7ç/Z] (ES+) 277 (100%, [[MH] +).] A mixture of crude [BENZHYDRYLIDENE] (3-fluoropyridin-2-yl) amine (49.4 g), 2-bromoacetaldehyde diethyl acetal (56 ml, 0.37 mol), 48% hydrobromic acid (20 ml) and water (20 ml) were heated to [90°C] for 20 min. On cooling, the mixture was diluted with isopropanol (450 ml), NaHCOs [(68] g) was added cautiously, and the mixture filtered. The residue was washed with further isopropanol (450 [ML)] and the combined organics were stirred and heated to [50°C] for 18 h. On cooling, the solution was concentrated in vacuo and azeotroped with EtOAc (2 x 440 ml). The remaining mixture was suspended in EtOAc (400 ml), filtered and the residue washed with further EtOAc until the filtrate ran clear [(11).] The solid orange-coloured residue was then suspended between water (100 ml) and EtOAc (220 ml) and the aqueous phase pH adjusted to 8-9 by addition of saturated aqueous NaHCO3 solution (450 ml). The phases were separated and the aqueous extracted with further EtOAc (2 x 220 ml). The combined organic extracts were dried over anhydrous [MGS04] and concentrated in vacuo affording crude [8-FLUOROIMIDAZO [1, 2-APYRIDINE.] Purification was achieved by dissolving the crude material in EtOAc (1 l) and extracting with 2 N hydrochloric acid (5 x 50 [ML).] The acid washings were back-extracted with EtOAc (3 x 100 ml), adjusted to pH 11-12 using 4 N aqueous sodium hydroxide solution and re-extracted with EtOAc (5 x 100 [ML).] The combined organic fractions were washed with brine, dried over anhydrous [MGS04] and concentrated [17] vacua to afford 8- [FLUOROIMIDAZO] [[L,] 2-a] pyridine (11.4 [G, 76%] from 2-chloro-3-fluoropyridine): [No.H ] [(400] MHz, CDCl3) 6.69-6. 74 [(1H,] m), 6.84-6. 87 [(1H,] m), 7.65-7. 66 (2H, m), 7.97 [(1H,] dd, J 1.0 and 6.8) [; M/Z (ES+)] 137 (100%, [[MH]] +). A solution of bromine in [MEOH,] saturated with [KBR] (0. 86 M, 1.0 ml, 0.86 mmol) was added to a cooled, stirred solution of 8-fluoroimidazo[1, 2- pyridine (118 mg, 0. 86 mmol) and NaOAc (85 mg, 1.04 mmol) in methanol saturated with [KBR] (1.6 ml). After 2 min, the solution was poured into water (50 [ML)] and the resulting yellow solution extracted with [CH2CL2] (3 x 50 ml). The combined organic fractions were dried over anhydrous [NA2SO4] and concentrated in vacuo, yielding 3-bromo-8- [FLUOROIMIDAZO] [1, 2-a] pyridine as a yellow solid (155 mg, 83%), used without further purification: [5H] (400 MHz, [CD13)] 6. [86-6.] 91 [(1H,] m), 6.95-6. 99 (1H, m), 7.65 [(1H,] s), 7.98 [(1H,] dd, [J 0.] 8 and 6.7) ; [M/Z] (ES+) 217 [(100%,] [[MH] +),] 215 (100). [3-BROMO-8-FLUOROIMIDAZO] [1, [2-A] PYRIDINE,] 3- [[3- (5,] 5-dimethyl- [1, 3,2] dioxaborinan-2-yl) phenyl] pyridine (prepared as described in WO [01/90108),] tetrakis (triphenylphosphine) palladium [(0)] (42 mg, 0.036 [MMOL),] THF (3.6 ml) and 2 M aqueous [NA2CO3] solution were combined and the vigorously stirred mixture heated to [75°C] for 16 h. After cooling, the mixture was partitioned between 1 N aqueous [NAOH] solution (20 ml) and [CHZCL2] (20 ml) and the aqueous phase extracted with further [CHZCLZ] (20 ml). The combined organics were dried over anhydrous [NA2SO4] and concentrated in vacuo, and the residue purified by column chromatography (silica; 2% [ETOH/ETOAC)] to afford the title imidazopyridine as a white amorphous solid (96 mg, 47%): [SN] (400 MHz; DMSO) 6.93-6. 98 [(1H,] m), 7.21-7. 24 [(1H,] m), 7.51-7. 54 [(1H,] m), 7.68-7. 76 (2H, m), 7. 82 [(1H,] t, J [1.] 6), 7.94 [(1H,] s), 8.01 [(1H,] t, J 1.6), 8. 19-8.22 [(1H,] m), 8.54 [(1H,] dd, [J 0.] 8 and 7.0), 8.62 [(1H,] dd, J 1.6 and 4.7), 9.02 [(1H,] dd, J 0. 8 and 2.3) ; [MILZ] (ES+) 290 (30%, [MH] +), 166 (100).
References:
MERCK SHARP & DOHME LIMITED WO2003/99816, 2003, A1 Location in patent:Page 42-43
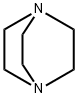
280-57-9
554 suppliers
$5.00/5g

372-47-4
365 suppliers
$5.00/1g
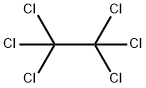
67-72-1
265 suppliers
$11.00/25g

17282-04-1
358 suppliers
$7.00/10g
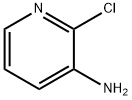
6298-19-7
488 suppliers
$6.00/5g

17282-04-1
358 suppliers
$7.00/10g

372-47-4
365 suppliers
$5.00/1g
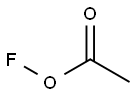
78948-09-1
2 suppliers
inquiry

2267-37-0
0 suppliers
inquiry

17282-04-1
358 suppliers
$7.00/10g
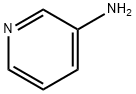
462-08-8
470 suppliers
$14.00/25g

17282-04-1
358 suppliers
$7.00/10g