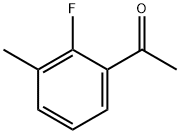
2'-Fluoro-3'-methylacetophenone synthesis
- Product Name:2'-Fluoro-3'-methylacetophenone
- CAS Number:865664-05-7
- Molecular formula:C9H9FO
- Molecular Weight:152.17
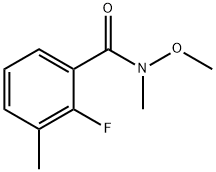
680610-60-0
1 suppliers
inquiry

75-16-1
266 suppliers
$12.00/10ml

865664-05-7
29 suppliers
$27.00/100mg
Yield:865664-05-7 96%
Reaction Conditions:
in tetrahydrofuran;diethyl ether at -78; for 1 h;
Steps:
Preparation of 1-(2-fluoro-3-methylphenyl)ethanone (456B)
To a solution of compound 456A (270 g, 1.37 mol) in THF (2.7 L) was added methyl magnesium bromide (3.0 M in Et2O, 1.82 L, 5.48 mol) drop wise at -78 °C. The resulting mixture was stirred for 1 h at -78 °C. The reaction mixture was then quenched with saturated ammonium chloride (5.0 L) and extracted with EtOAc (2 x 5.0 L). The combined organic layers were dried over sodium sulfate and concentrated under reduced pressure. The mixture thus obtained was purified by silica gel chromatography (10% EtOAc in hexanes) to give compound 456B (200 g, 96%). 1H NMR (400 MHz, Chloroform-d) δ 7.68 - 7.64 (m, 1 H), 7.37 - 7.34 (m, 1 H), 7.09 (t, J = 7.0 Hz, 1 H), 2.64 (s, 3H), 2.32 (s, 3H).
References:
WO2016/22724,2016,A1 Location in patent:Page/Page column 332
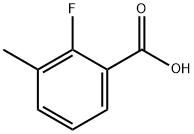
315-31-1
221 suppliers
$8.00/250mg

865664-05-7
29 suppliers
$27.00/100mg