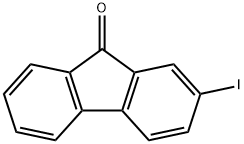
2-IODO-9H-FLUOREN-9-ONE synthesis
- Product Name:2-IODO-9H-FLUOREN-9-ONE
- CAS Number:3096-46-6
- Molecular formula:C13H7IO
- Molecular Weight:306.1
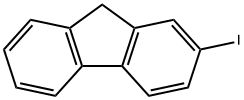
2523-42-4
197 suppliers
$6.00/250mg

3096-46-6
17 suppliers
$106.00/50mg
Yield:3096-46-6 95%
Reaction Conditions:
with tert.-butylhydroperoxide;1-n-butyl-3-methylimidazolim bromide in water at 55; for 20 h;
Steps:
General procedure for oxygenation of benzylic C-H bonds
General procedure: Charged 0.5 mmol of 3-(9H-fluoren-2-yl)prop-2-yn-1-ol 13a (110 mg, 0.5 mmol), 0.5 mL of [bmim]Br and TBHP (0.5 mL, 3.6 mmol) were taken in a round bottom flask and heated to 55 °C until completion of the reaction. The reaction course was monitored by TLC. After completion of the reaction, the reaction mixture was extracted using ethyl acetate (3 * 4 mL). The organic layer was concentrated under vacuum and purified by column chromatography on silica gel to afford the desired product as 2-(3-hydroxyprop-1-yn-1-yl)-9H-fluoren-9-one 13b Yellow Solid;
References:
Naidu, Shivaji;Reddy, Sabbasani Rajasekhara [Journal of Molecular Liquids,2016,vol. 222,p. 441 - 445]
3096-57-9
120 suppliers
$45.00/50mg

3096-46-6
17 suppliers
$106.00/50mg
486-25-9
459 suppliers
$5.00/25g

3096-46-6
17 suppliers
$106.00/50mg

486-25-9
459 suppliers
$5.00/25g

16218-30-7
14 suppliers
$106.00/100mg

3096-46-6
17 suppliers
$106.00/50mg
607-57-8
156 suppliers
$16.00/5g

3096-46-6
17 suppliers
$106.00/50mg