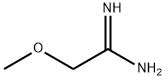
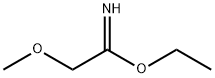
2-METHOXY-ACETAMIDINE synthesis
- Product Name:2-METHOXY-ACETAMIDINE
- CAS Number:3122-73-4
- Molecular formula:C3H8N2O
- Molecular Weight:88.11
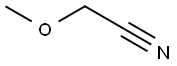
1738-36-9
148 suppliers
$20.00/5g

3122-73-4
14 suppliers
$215.61/1gm:
Yield: 88%
Reaction Conditions:
Stage #1:2-methoxyacetonitrile with sodium methylate in methanol at 20; for 48 h;
Stage #2: with ammonium chloride in methanol for 12 h;
Steps:
A.A-4.1
A-4: 2-Methoxymethyl-5-(pyrimidin-5-ylamino)-pyrimidine-4-carboxylic acid methyl esterStep 1: 2-Methoxy-acetamidineTo a solution of methoxy-acetonitrile (6 g, 84.4 mmol) in MeOH (60 ml) was added sodium methylate (0.86 g, 16.0 mmol) and the reaction mixture was stirred at ambient temperature for 48 hours. Ammonium chloride (4.52 g, 84.5 mmol) was added to the reaction mixture and stirring was continued for another 12 h. The solvent was removed under reduced pressure yielding the title compound which was used in the next step without any further purification. Yield: 6.5 g (88%)MS: M=89.1 (M+H)+
References:
Bleicher, Konrad;Flohr, Alexander;Zbinden, Katrin Groebke;Koerner, Matthias;Kuhn, Bernd;Peters, Jens-Uwe;Rodriguez-Sarmiento, Rosa Maria;Vieira, Eric US2011/183979, 2011, A1 Location in patent:Page/Page column 18

1738-36-9
148 suppliers
$20.00/5g
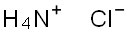
12125-02-9
1247 suppliers
$10.00/250G

3122-73-4
14 suppliers
$215.61/1gm:
42945-65-3
11 suppliers
$45.00/5mg

3122-73-4
14 suppliers
$215.61/1gm:
88512-05-4
3 suppliers
inquiry

3122-73-4
14 suppliers
$215.61/1gm: