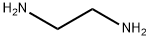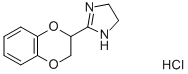
IDAZOXAN HYDROCHLORIDE synthesis
- Product Name:IDAZOXAN HYDROCHLORIDE
- CAS Number:79944-56-2
- Molecular formula:C11H13ClN2O2
- Molecular Weight:240.69
Yield: 81%
Reaction Conditions:
Stage #1:2-cyano-1,4-benzodioxane with hydrogenchloride;sodium methylate in methanol at 20; for 4 h;
Stage #2:ethylenediamine with hydrogenchloride in methanol at 0 - 10; for 20 h;
Steps:
5.1
5.1. Processes for the Synthesis of Idazoxan Hydrochloride of the Prior Art Idazoxan hydrochloride can be synthesized according to the published method described below: Preparation of 2-[2-(1,4-benzodioxanyl)]-2-imidazoline hydrochloride. A solution of sodium methoxide (1.45 g) in methanol (20 ml) is added in the space of one minute to a stirred solution of 2-cyano-1,4-benzodioxane (145 g) in methanol (870 ml) at ambient temperature. After stirring for a further 4 hours at ambient temperature, the solution is cooled and ethylenediamine (64.7 g) is added dropwise at a temperature of 5° C. A solution of hydrogen chloride in methanol (134 g of solution comprising 34.8 g of hydrogen chloride) is then added to the stirred solution in the space of 2 hours and at a temperature of 5° C. After a further 20 hours at 0-10° C., the precipitated ethylenediamine dihydrochloride is removed by filtration and the filtrate is reduced to 300 g under vacuum at 40° C. Ethylenediamine dihydrochloride is again removed and the remaining filtrate is subjected to evaporation under vacuum at 40° C. to complete dryness. The solid residue (225 g) is stirred with dichloromethane (1.1 litres) and dry hydrogen chloride is sparged in at 5-10° C. until a slight excess is obtained. The crude product is subsequently removed by filtration (172 g) and combined with a second crop (24 g) obtained by concentrating the filtrate under vacuum at 40° C. The crystallization of these two crops from ethanol with hot filtration and concentration of the filtrate under vacuum until 384 g are obtained gives an off-white crystalline product (175.5 g, 81%), melting point 207-208° C. If the ethylenediamine and the hydrogen chloride are added to the methanol in the reverse order, a similar yield is obtained.
References:
Bougaret, Joel;Avan, Jean-Louis;Segonds, Roland US2005/90537, 2005, A1 Location in patent:Page/Page column 8; 9
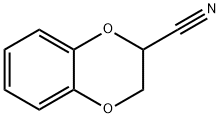
1008-92-0
50 suppliers
inquiry

79944-56-2
67 suppliers
$95.90/50mg
120-80-9
500 suppliers
$13.00/25g

79944-56-2
67 suppliers
$95.90/50mg