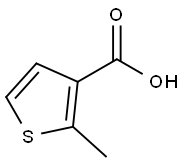
2-METHYL-THIOPHENE-3-CARBOXYLIC ACID synthesis
- Product Name:2-METHYL-THIOPHENE-3-CARBOXYLIC ACID
- CAS Number:1918-78-1
- Molecular formula:C6H6O2S
- Molecular Weight:142.18

74-88-4
339 suppliers
$15.00/10g

1918-78-1
83 suppliers
$9.00/250mg
Yield: 20%
Reaction Conditions:
Stage #1:3-Thiophene carboxylic acid with n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -78 - 0; for 1.16667 h;
Stage #2:methyl iodide in tetrahydrofuran;hexane at -78 - 20; for 1 h;
Stage #3: with hydrogenchloride;water in tetrahydrofuran;hexane
Steps:
27.a
EXAMPLE 27; Preparation of 2-Amino-5-(2-methylthien-3-yl)-3-methyl-5-(3-pyrimidin-5-ylphenyl)-3,5-dihydro-4H-imidazol-4-one; Step a); Preparation of Compound 19; A solution of diisopropylamine (8.28 g, 81.8 mmol) in THF (190 mL) at -20° C. was treated with n-butyllithium (52 mL of a 1.6 M solution in hexanes, 83.2 mmol) over a period of 20 min, then slowly warmed to 0° C. under stirring for 30 min. This solution was cooled to -78° C. and treated dropwise with a solution of thiophene-3-carboxylic acid (4.99 g, 38.9 mmol) in THF (40 mL) over a period of 10 min. The mixture was stirred at -78° C. for an additional 10 min, then treated with iodomethane (6.16 g, 43.3 mmol) and stirred for 1 h, allowing the solution to slowly warm to rt. Water (200 mL) was added, and the mixture was made acidic by adding 2 N HCl (40 mL). The layers were separated and the aqueous layer was extracted with ether (3×75 mL), then the combined organic layers were dried over sodium sulfate, filtered and concentrated. Purification by flash chromatography (silica, 50:50:1 ethyl acetate/hexanes/acetic acid) afforded 19 (1.12 g, 20%) as a white solid: 1H NMR (500 MHz, CDCl3) δ 7.45 (d, J=5.4 Hz, 1H), 7.01 (t, J=5.4 Hz, 1H), 2.77 (s, 3H).
References:
Location in patent:Page/Page column 18-19

60-29-7
7 suppliers
$30.00/50g
88-13-1
365 suppliers
$5.00/1g

74-88-4
339 suppliers
$15.00/10g

1918-78-1
83 suppliers
$9.00/250mg
19432-66-7
62 suppliers
$45.00/1g

1918-78-1
83 suppliers
$9.00/250mg
30319-05-2
75 suppliers
$28.00/1g

124-38-9
122 suppliers
$175.00/23402

1918-78-1
83 suppliers
$9.00/250mg