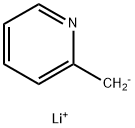
(2-pyridylmethyl)lithium synthesis
- Product Name:(2-pyridylmethyl)lithium
- CAS Number:1749-29-7
- Molecular formula:C6H6LiN
- Molecular Weight:99.06
Yield:-
Reaction Conditions:
in tetrahydrofuran;hexanes at -20; for 1.75 h;
Steps:
45.1
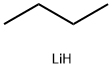
To a -20° C. solution of 2-picoline (0.687 g, 7.38 mmol) in anhydrous THF (5 ml) was slowly added BuLi (5.54 ml of 1.6M soln. in hexanes, 8.86 mmol), resulting in a dark orange solution. The reaction mixture was allowed to stir for 105 min at -20° C. The chilled reaction mixture was cannulated over a period of 2 h into a -40° C. solution of 39 (1.79 g, 6.2 mmol) in anhydrous THF (10 ml). The reaction mixture was allowed to stir for 2 h at -40° C., then was allowed to warm to rt. Saturated aqueous NH4Cl (20 ml), was added and the mixture was extracted with CH2Cl2 (2×40 ml). The organic layer was separated and dried over Na2SO4, followed by concentration and flash chromatography (0-20% acetone/CH2Cl2) to afford of 74 (0.968 g, 6.2 mmol; 41%) as a clear oil.
References:
US2007/10513,2007,A1 Location in patent:Page/Page column 28-29
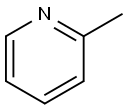
109-06-8
394 suppliers
$16.00/25g

1749-29-7
2 suppliers
inquiry

109-06-8
394 suppliers
$16.00/25g
4111-54-0
323 suppliers
$59.79/4x25ml

1749-29-7
2 suppliers
inquiry
108-18-9
353 suppliers
$10.00/1g