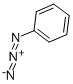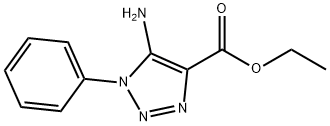
5-Amino-1-phenyl-1H-1,2,3-triazole-4-carboxylic acid ethyl
ester synthesis
- Product Name:5-Amino-1-phenyl-1H-1,2,3-triazole-4-carboxylic acid ethyl
ester - CAS Number:20271-37-8
- Molecular formula:C11H12N4O2
- Molecular Weight:232.24
Yield:20271-37-8 62%
Reaction Conditions:
with sodium ethanolate in ethanol at 20;Inert atmosphere;Temperature;
Steps:
2.1.1 General procedure for exclusive obtaining ethyl 5-amino-1-phenyl-1H-triazole-4-carboxylates 16a-i
General procedure: A mixture of arylazide (15a-i, 0.01mol) and ethyl cyanoacetate (0.01mol) in 25mL of dry ethanol containing 0.01mol of sodium ethoxide was kept under stirring at room temperature in inert atmosphere during three hours. The reaction mixture was poured into 50mL of ice water. The precipitate that formed was collected by filtration and washed with water to obtain 16a-i. Ethyl 5-amino-1-phenyl-1H-1,2,3-triazole-4-carboxylate (16a). 62% yield. m.p.: 130-131°C (lit. [37] 130-131°C); IR (KBr, cm-1): 3422-3312, 2986, 1677, 1626, 1513, 1278-1259, 1109, 755-691; 1H NMR (500.00MHz, DMSO-d6, TMS, δ ppm): 0.84 (t, 3H, J 7.1Hz), 3.84 (q, 2H, J 7.1Hz), 5.86 (s, 2H), 6.91 (t, 1H, J 4.4Hz), 7.19-7.05 (m, 5H); HRMS (ESI) calc. for C11H12N4NaO2 255.0858, found [M+Na]+ 255.0844. Δ 5.5ppm.
References:
Silva, Thais B.;Ji, Kathya N.K.;Petzold Pauli, Fernanda;Galv?o, Raíssa M.S.;Faria, Ana F.M.;Bello, Murilo L.;Resende, Jackson A.L.C.;Campos, Vinicius R.;Forezi, Luana da S.M.;da Silva, Fernando de C.;Faria, Robson X.;Ferreira, Vitor F. [Bioorganic Chemistry,2021,vol. 116,art. no. 105250]
13621-50-6
85 suppliers
$22.00/100mg

20271-37-8
8 suppliers
inquiry

5873-88-1
0 suppliers
inquiry

20271-37-8
8 suppliers
inquiry

62-53-3
629 suppliers
$10.00/1g

20271-37-8
8 suppliers
inquiry

53591-51-8
0 suppliers
inquiry

20271-37-8
8 suppliers
inquiry