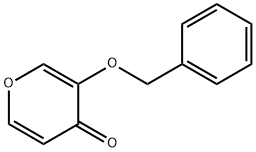
3-(benzyloxy)-4H-pyran-4-one synthesis
- Product Name:3-(benzyloxy)-4H-pyran-4-one
- CAS Number:61049-67-0
- Molecular formula:C12H10O3
- Molecular Weight:202.21
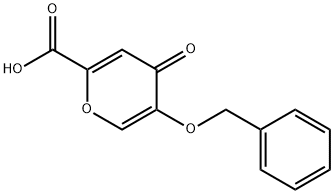
1219-33-6
58 suppliers
$72.00/100mg

61049-67-0
42 suppliers
$34.00/100mg
Yield: 70%
Reaction Conditions:
in 1-methyl-pyrrolidin-2-one for 4 h;Reflux;
Steps:
11 3-Benzyloxy-4H-pyran-4-one (14b)
5-Benzyloxy-4-oxo-4H-pyran-2-carboxylic acid (13b) (15.87 g, 64.5 mmol) and 1-methyl-2- pyrrolidinone (160 mL) were refluxed for 4 h.
Then the solvent was removed azeotropically with N,N-dimethylformamide (160 mL) in high vacuum.
The residue was extracted into dichloromethane and washed with 5% aqueous sodium hydroxide (3 * 90 mL).
The organic layer was dried over Na2SO4, concentrated and recrystallized from toluene to give a pale yellow solid (14b) (9.07 g, 70%).
References:
Xie, Yuan-Yuan;Lu, Zidong;Kong, Xiao-Le;Zhou, Tao;Bansal, Sukhi;Hider, Robert [European Journal of Medicinal Chemistry,2016,vol. 115,p. 132 - 140]
496-63-9
139 suppliers
$12.00/100mg
100-39-0
424 suppliers
$10.00/10 g

61049-67-0
42 suppliers
$34.00/100mg
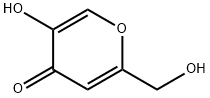
501-30-4
610 suppliers
$5.00/25mg

61049-67-0
42 suppliers
$34.00/100mg

15771-06-9
57 suppliers
$25.00/250mg

61049-67-0
42 suppliers
$34.00/100mg
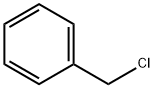
100-44-7
627 suppliers
$13.50/250G

61049-67-0
42 suppliers
$34.00/100mg