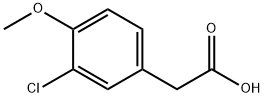
3-chloro-4-methoxyphenylacetic acid synthesis
- Product Name:3-chloro-4-methoxyphenylacetic acid
- CAS Number:13721-20-5
- Molecular formula:C9H9ClO3
- Molecular Weight:200.62
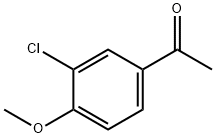
37612-52-5
73 suppliers
$23.00/1g

13721-20-5
60 suppliers
$60.00/100mg
Yield: 84%
Reaction Conditions:
with sodium hydroxide;lead(IV) tetraacetate in 1,4-dioxane;methanol;water;toluene
Steps:
7.2 3-(3-Chloro-4-methoxyphenyl)-5-methyl-4-[4-methylsulfonylphenyl]isoxazole
Step 2. Preparation of 3-chloro-4-methoxyphenylacetic acid. A mixture of 3-chloro-4-methoxyacetophenone from Step 1 (10.0 g. 54.2 mmol) and boron trifluoride etherate complex (26.6 mL, 0.216 mol) in 20 mL of methanol was added to a suspension of lead tetraacetate (24 g, 54.2 mmol) in 50 mL of toluene. The mixture was stirred at room temperature for 16 hours, treated with 50 mL of water. The phases were separated and the aqueous phase was washed with toluene. The toluene solution was dried over anhydrous MgSO4, filtered and concentrated in vacuo to provide an oil which was dissolved in 40 mL of dioxane and treated with excess 2.5N sodium hydroxide solution. The solution was stirred at room temperature for 2 hours and concentrated in vacuo. The residue was extracted with dichloromethane and the aqueous phase was acidified with concentrated HCl. The acidic solution was extracted with dichloromethane. The dichloromethane extract was dried over anhydrous MgSO4, filtered and concentrated in vacuo to afford pure 3-chloro-4-methoxyphenylacetic acid (9.11 g, 84%) which was used directly in the next step.
References:
G. D. Searle & Co. US5859257, 1999, A

13719-57-8
19 suppliers
inquiry
201230-82-2
1 suppliers
inquiry

13721-20-5
60 suppliers
$60.00/100mg
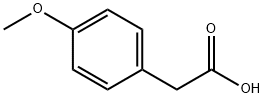
104-01-8
508 suppliers
$6.00/10g

13721-20-5
60 suppliers
$60.00/100mg

7569-58-6
25 suppliers
inquiry

13721-20-5
60 suppliers
$60.00/100mg

13719-57-8
19 suppliers
inquiry

13721-20-5
60 suppliers
$60.00/100mg