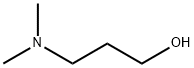
3-Dimethylamino-1-propanol synthesis
- Product Name:3-Dimethylamino-1-propanol
- CAS Number:3179-63-3
- Molecular formula:C5H13NO
- Molecular Weight:103.16

124-40-3
502 suppliers
$18.00/100ml
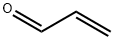
107-02-8
4 suppliers
$38.60/4s8501

3179-63-3
184 suppliers
$10.60/1gm:
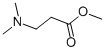
109-55-7
335 suppliers
$13.00/25mL
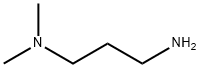
Yield:109-55-7 80.3 %Chromat. ,3179-63-3 13.9 %Chromat.
Reaction Conditions:
Stage #1:dimethyl amine;acrolein at 4; for 1.25 h;autoclave;
Stage #2: with ammonia;hydrogen;Raney cobalt in water at 80; under 45004.5 Torr; for 2 h;Product distribution / selectivity;autoclave;
Steps:
2
EXAMPLE 2; A 270 ml autoclave was initially charged with 67.6 g of dimethylamine (1.5 mol), and 16.8 g of acrolein (0.3 mol) were pumped in while cooling (4° C.) over 60 minutes. The mixture was stirred for 15 minutes. A sample was taken and analyzed by means of gas chromatography. Then the contents of this autoclave were transferred by means of a connecting line, by injection of hydrogen, into a 270 ml high-pressure autoclave which had already been initially charged with 1.8 g of Ra-Co (THF-washed) in 51.0 g of NH3 (3.0 mol) and 10.8 g of water (0.6 mol). The second autoclave was heated to 80° C., and hydrogen was injected to 60 bar. Then hydrogenation was effected for 2 hours and the pressure was maintained by adding hydrogen. After 2 hours, a sample was taken and analyzed by means of gas chromatography. The chromatogram showed full conversion of acrolein and of the enamine which occurred as the intermediate, and a DMAPA yield of 80.3%. The dimethylaminopropanol (DMAPOL) yield was 13.9%. The yields of the compounds were determined by means of gas chromatography as area percentages (a %).
References:
BASF SE US2011/60166, 2011, A1 Location in patent:Page/Page column 8
3853-06-3
35 suppliers
$148.00/25g

3179-63-3
184 suppliers
$10.60/1gm:
506-59-2
517 suppliers
$5.00/5 g

124-40-3
502 suppliers
$18.00/100ml

109-78-4
342 suppliers
$10.00/1g

3179-63-3
184 suppliers
$10.60/1gm:

124-40-3
502 suppliers
$18.00/100ml

109-78-4
342 suppliers
$10.00/1g

3179-63-3
184 suppliers
$10.60/1gm:
20120-21-2
46 suppliers
$48.89/5g

3179-63-3
184 suppliers
$10.60/1gm: