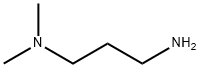
3-Dimethylaminopropylamine synthesis
- Product Name:3-Dimethylaminopropylamine
- CAS Number:109-55-7
- Molecular formula:C5H14N2
- Molecular Weight:102.18
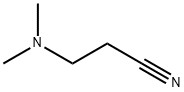
1738-25-6
120 suppliers
$30.00/25mL

109-55-7
335 suppliers
$13.00/25mL
Yield:109-55-7 99.98%
Reaction Conditions:
with potassium hydroxide;sodium hydroxide;hydrogen;Ni-MC502 in water at 90; under 5932.2 Torr;
Steps:
2; 3.6 Example 2; Hydrogenation procedure
Example 2Hydrogenation Procedure [00045] A one-liter autoclave reactor equipped with double turbine blades, dispersimax-type agitator, a coil extending to the bottom to circulate the transfer fluid from a temperature controlled bath for temperature control, and a fritted, stainless steel metal sample port below the liquid level is used to react hydrogen with 3-(dimethylamino)propionitrile. Hydrogen is fed from a cylinder equipped with a pressure gauge and a regulator to add hydrogen to the reactor when the pressure drops below the set pressure. The hydrogen flows though a mass flow meter. The 3-(dimethylamino)propionitrile (Acros) is pumped to the autoclave with an Isco Model 500D syringe pump. To the autoclave is charged 37.5 grams of sponge nickel catalyst (Degussa MC502) with iron and chromium added to promote the hydrogenation reaction (the catalyst contains about 85% nickel, 10% aluminum, 2% chromium, and 2% iron). The catalyst is washed 3 times with water and 3 times with 3-dimethylaminopropylamine (Acros; contaminated with 72 ppm TMPDA by GC analysis), each wash consisting of mixed catalyst and material in a 100 L graduated cylinder, settling. the catalyst and decanting the top 50 mL of clear liquid. The catalyst, water, and 3-dimethylaminopropylamine slurry amounting to 50 mL are then charged to the autoclave. Additionally, 265 mL of 100% 3-dimethylaminopropylamine and 6 mL of 25% (wt.) caustic solution in water is charged. The caustic solution is a blend containing 50 wt. % sodium hydroxide and 50 wt. % potassium hydroxide. The agitator is turned on, and the autoclave heated to 60 C. The autoclave is then purged three times with nitrogen, and then three times with hydrogen, before being pressurized to 7.805 atm with hydrogen. The autoclave is then heated to 90 C., and the pressured checked and maintained for 5 minutes. [00046] The feed of 3-(dimethylamino)propionitrile containing 0.04 wt. % water is then started to the autoclave at a rate of 5 mL/minute using the syringe pump. Pressure and temperature are maintained at 7.805 atm and 90 C., respectively, during the entirety of the run. After 27 minutes, the feed is stopped, and a 150 g sample is withdrawn from the autoclave for analysis. The feed is then resumed under the same conditions as before. This procedure is then repeated for a total of 7 cycles. [00047] The reaction mixture was sampled after each cycle and analyzed for purity, reaction progress, and the presence and amount of by-products (if any) formed. Analysis was by gas chromatography (HP 5890 Series II; Phenomenex Zebron ZB-1 capillary column, Phenomenex Cat. No. 7HK-G001-36) with flame ionization detection in order to quantify the by-product impurities.
References:
Solutia Inc. US6660887, 2003, B1 Location in patent:Page column 7

5350-67-4
9 suppliers
inquiry
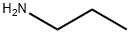
107-10-8
352 suppliers
$10.00/20mg
110-95-2
278 suppliers
$8.00/10g

109-55-7
335 suppliers
$13.00/25mL
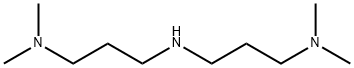
6711-48-4
180 suppliers
$8.00/25g
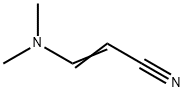
2407-68-3
83 suppliers
$36.00/1 g

109-55-7
335 suppliers
$13.00/25mL
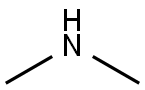
124-40-3
500 suppliers
$18.00/100ml
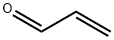
107-02-8
4 suppliers
$38.60/4s8501

109-55-7
335 suppliers
$13.00/25mL