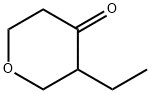
3-Ethyltetrahydro-4H-pyran-4-one synthesis
- Product Name:3-Ethyltetrahydro-4H-pyran-4-one
- CAS Number:21398-42-5
- Molecular formula:C7H12O2
- Molecular Weight:128.17
Yield:21398-42-5 39.1%
Reaction Conditions:
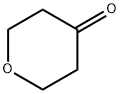
Stage #1: Tetrahydro-4H-pyran-4-onewith lithium diisopropyl amide in tetrahydrofuran;N,N,N,N,N,N-hexamethylphosphoric triamide at -78; for 0.5 h;Inert atmosphere;
Stage #2: ethyl iodide in tetrahydrofuran;N,N,N,N,N,N-hexamethylphosphoric triamide at -78 - 20; for 12 h;
Steps:
65.A 3-ethyldihydro-2H-pyran-4(3H)-one
Example 65A
3-ethyldihydro-2H-pyran-4(3H)-one
To a mixture of tetrahydropyran-4 one (5 g, 0.05 mol) and hexamethylphosphoramide (9 mL) in tetrahydrofuran (100 mL) was added lithium diisopropylamide (34.7 mL, 0.062 mol) dropwise at -78° C. under N2.
After the addition the mixture was stirred for 0.5 h at -78° C. Iodoethane (7.78 g, 0.199 mol) was added to the mixture dropwise.
The ddresulting mixture was stirred for 12 hours at room temperature. TLC (petroleum ether:ethyl acetate=4:1) indicated the reaction was not complete, the mixture was quenched with aqueous ammonium chloride (100 mL), extracted with ethyl acetate (3*50 mL), the combined organic layers was concentrated in vacuo and the residue was purified by chromatography on silica using petroleum ether: ethyl acetate from 15:1 to 10:1 to yield compound the title compound (2.5 g, 39.1%) as a colorless liquid. 1H NMR (400 MHz, CD3OD) δ (ppm): 4.15 (m, 2H), 3.80 (m, 1H), 3.45 (m, 1H), 2.58 (m, 1H), 2.48 (m, 2H), 1.81 (m, 1H), 1.30 (m, 1H), 0.92 (m, 3H).
References:
US2013/261129,2013,A1 Location in patent:Paragraph 0383