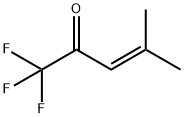
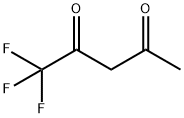
3-Penten-2-one, 1,1,1-trifluoro-4-methyl- synthesis
- Product Name:3-Penten-2-one, 1,1,1-trifluoro-4-methyl-
- CAS Number:400-31-7
- Molecular formula:C6H7F3O
- Molecular Weight:152.11
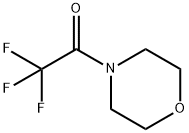
360-95-2
92 suppliers
$10.00/250mg
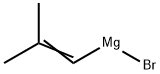
38614-36-7
70 suppliers
$172.00/100ml

400-31-7
9 suppliers
inquiry
Yield:400-31-7 68%
Reaction Conditions:
Stage #1: 2,2,2-Trifluoro-1-morpholin-4-yl-ethanone;2-methylpropen-1-ylmagnesium bromide in tetrahydrofuran at 0 - 22; for 1.33 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;water at 0 - 30;
Steps:
1
2-Methyl-l-propenylmagnesium bromide (0.5M in THF, 2.4 L, 1.2 mol) was cooled to 00C. N-trifluoroacetylmorpholine (198.1 g, 1.082 mol) was added dropwise over 20 minutes to the Grignard solution at a rate such that the temperature did not exceed 100C. The reaction mixture was allowed to stir at 15°C-22°C for 1 hour. The reaction mixture was cooled to 00C and treated dropwise with 300 mL of concentrated hydrochloric acid, keeping the temperature below 300C. The reaction was further diluted with 900 mL of water and 700 mL of dodecane, and the layers were cut. The organic phase was washed four times with a solution of 1.1 L of water and 300 mL of methanol, then with 1.2 L of water, and finally dried over 100 g of 4 A molecular sieves for 16 hours. The solution was filtered away from the molecular sieves and distilled at 150 mmHg (bath temperature up to 1100C) to give l,l,l-trifluoro-4-methyl-3-penten-2-one (699.3 g, 16 wt.%, thus 111.9 g 1 , 1 , 1 -trifluoro-4- methyl-3-penten-2-one, 68% yield) as a solution in THF.
References:
WO2010/141331,2010,A2 Location in patent:Page/Page column 14-15

38614-36-7
70 suppliers
$172.00/100ml
104863-67-4
141 suppliers
$5.00/250mg

400-31-7
9 suppliers
inquiry
367-57-7
276 suppliers
$10.00/2g

75-16-1
266 suppliers
$12.00/10ml

400-31-7
9 suppliers
inquiry

367-58-8
0 suppliers
inquiry
