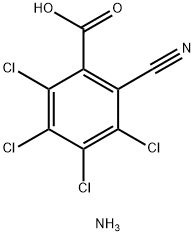
3,4,5,6-Tetrachloro-2-cyanobenzoic acid ammonium salt synthesis
- Product Name:3,4,5,6-Tetrachloro-2-cyanobenzoic acid ammonium salt
- CAS Number:34643-39-5
- Molecular formula:C8H4Cl4N2O2
- Molecular Weight:301.93

99651-72-6
0 suppliers
inquiry

34643-39-5
28 suppliers
inquiry
Yield:-
Reaction Conditions:
with ammonia;water in xylene at -4 - 85; for 6 h;Product distribution / selectivity;
Steps:
1.b; 5; 6
352 gm of TCPC solution obtained in step 1 (a) in the form of organic (xylene) layer was added drop by drop in a three necked round bottom flask containing 633 gm 25% liquor ammonia at molar ratio Jj9. Maintained at a temperature of 4.8°C. The flask was placed in an ice bath containing brine solution at temperature between -2°C to -4°C. The reaction mixture was allowed to stand for 60 minutes in the cold bath. The temperature was periodically checked to ensure that the temperature of the reaction mixture did not exceed 50C. Then the flask was removed from the bath and the temperature was slowly raised up to 500C in 1 hour time. The flask was further gradually heated to 85°C and stirred at this temperature for 4 hours. A hazy reaction mass was obtained. This mass was filtered in a Buchner funnel containing filter paper. Wet cake obtained was washed with 200 ml xylene. Xylene washed cake was dissolved in hot water at 800C to form a clear solution; to this solution, 100 ml of xylene was
References:
WO2008/96371,2008,A1 Location in patent:Page/Page column 13-16