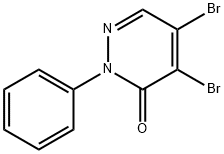
4,5-DIBROMO-2-PHENYL-2,3-DIHYDROPYRIDAZIN-3-ONE synthesis
- Product Name:4,5-DIBROMO-2-PHENYL-2,3-DIHYDROPYRIDAZIN-3-ONE
- CAS Number:14305-08-9
- Molecular formula:C10H6Br2N2O
- Molecular Weight:329.98
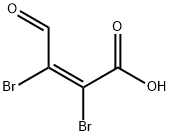
488-11-9
257 suppliers
$5.00/5g
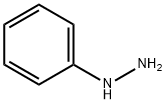
100-63-0
358 suppliers
$10.00/1g

14305-08-9
65 suppliers
$6.00/250mg
Yield:-
Reaction Conditions:
with hydrogenchloride in water at 70; for 18 h;
Steps:
3 EXAMPLE 3; 4,5-dibromo-2-phenylpyridazin-3(2H)-one
Mucobromic acid (10.0 g, 38.8 mmol) was dissolved in 250 ml of 6N HCl in a 500 ml round bottom flask at room temperature. Phenyl hydrazine (4.58 ml, 46.6 mmol) was dissolved in 100 ml of 6 N HCl and added to the reaction with vigorous stirring at 70° C. for 18 hours. An off-white precipitate formed immediately. After 18 hours, LC/MS showed reaction completion. The reaction was allowed to partially cool. 100 ml of ethyl acetate was then added in an attempt to extract the product. The precipitate went into solution, but the solution was homogenous and not two layers as expected. The reaction was allowed to stand in an attempt to allow the two layers to separate. As the reaction cooled, a large amount of precipitate formed. It was found that the HCl converted the ethyl acetate to ethanol and acetic acid, which caused the solution to become homogenous and caused product precipitation. The solid was filtered, washed with diethyl ether and dried under vacuum to afford 10.54 g of an off-white solid. 1H NMR (300 MHz, CDCl3) δ 7.96 (s, 1H), 7.60-7.42 (m, 5H); LC/MS, tr=2.35 minutes (5 to 95% acetonitrile/water over 5 minutes at 1 ml/min, at 254 nm, at 50° C.), (M+H), Calculated=329, Found=329; HR/MS (M+H), Calculated=328.8920, Found=328.8927 (Δ mmu=0.7).
References:
Pharmacia Corporation US2005/20594, 2005, A1 Location in patent:Page 33
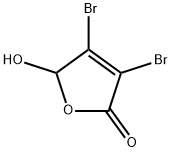
766-38-1
21 suppliers
$176.22/1g
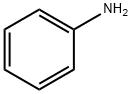
62-53-3
625 suppliers
$10.00/1g

14305-08-9
65 suppliers
$6.00/250mg

100-63-0
358 suppliers
$10.00/1g

14305-08-9
65 suppliers
$6.00/250mg