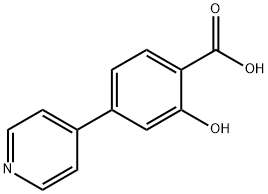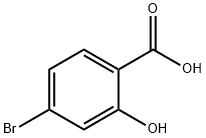
4-Bromo-2-hydroxybenzoic acid synthesis
- Product Name:4-Bromo-2-hydroxybenzoic acid
- CAS Number:1666-28-0
- Molecular formula:C7H5BrO3
- Molecular Weight:217.02
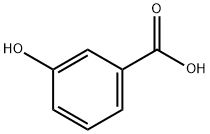
99-06-9
464 suppliers
$5.00/25g

1666-28-0
211 suppliers
$9.00/1g
Yield:1666-28-0 100%
Reaction Conditions:
with sulfuric acid;bromine in acetic acid
Steps:
I.92 [5-(4-Bromo-3-methoxy-phenyl)-3-methyl-2,3-dihydro-[1,3,4]thiadiazol-2-yl]-cyclohexyl-amine
[5-(4-Bromo-3-methoxy-phenyl)-3-methyl-2,3-dihydro-[1,3,4]thiadiazol-2-yl]-cyclohexyl-amine To a mixture of 3-hydroxybenzoic acid (14.480 mmol, 2 g) in acetic acid (14.5 mL) and sulfuric acid (1.5 mL) at 50° C., a solution of bromine (15.204 mmol, 0.780 mL) in acetic acid (7.2 mL) was added and stirred 30 minutes at 100° C. The reaction was allowed to cool to RT and diluted with water. The aqueous layer was extracted with ethyl acetate, washed with water and brine, dried (MgSO4), filtered, and concentrated under reduced pressure to give the 4-bromo-2-hydroxy-benzoic acid. Yield: 100%.
References:
Vergne, Fabrice;Ducrot, Pierre;Andrianjara, Charles;Bernardelli, Patrick;Lorthois, Edwige US2003/45557, 2003, A1
65-49-6
467 suppliers
$6.00/25g

1666-28-0
211 suppliers
$9.00/1g
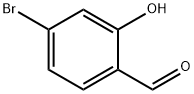
22532-62-3
299 suppliers
$5.00/250mg

1666-28-0
211 suppliers
$9.00/1g
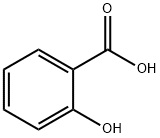
69-72-7
1195 suppliers
$5.00/10g

1666-28-0
211 suppliers
$9.00/1g