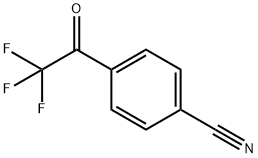
4'-CYANO-2,2,2-TRIFLUOROACETOPHENONE synthesis
- Product Name:4'-CYANO-2,2,2-TRIFLUOROACETOPHENONE
- CAS Number:23516-85-0
- Molecular formula:C9H4F3NO
- Molecular Weight:199.13
Yield: 51%
Reaction Conditions:
with cesium fluoride in tetrahydrofuran at 20; for 1 h;
Steps:
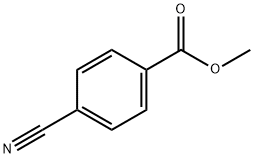
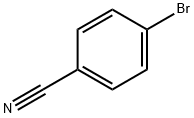
1 Method 21. step 1. 4-(2.2.2-Trifluoroacetyl)benzonitrile:
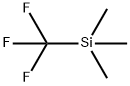
To a stirred solution of methyl 4-cyanobenzoate (1.5 g, 9.31 mmol) in dry THF (30 ml) was added trifluoromethyltrimethylsilane (1.98 g, 13.97 mmol) and cesium fluoride (0.14 g, 0.93 mmol) at room temperature and the reaction mixture was stirred for one hour. The pH of the reaction mixture was adjusted to 5-6 with IN HC1 and the aqueous layer was extracted with ethyl acetate (2 x 50 ml). The combined organic layers were washed with brine (50 ml), dried over anhydrous sodium sulfate and concentrated under reduced pressure. To the resulting residue, TBAF (9.31 ml, 1 M in THF, 9.31 mmol) and water (10 ml) was added at room temperature. The reaction mixture was stirred for one hour. Water (50 ml) was added and it the mixture was extracted with ethyl acetate (2 x 50 ml). The combined organic layers were washed with brine (50 ml), dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was purified by silica gel chromatography to afford the title compound (1 g, 51 %). 1H NMR (400 MHz, DMSO-d6): d 7.77 (d, / = 8.4 Hz, 2H), 7.90 (d, / = 8.0 Hz, 2H).
References:
CONSTELLATION PHARMACEUTICALS, INC.;WILSON, Jonathan, E.;BRUCELLE, Francois;LEVELL, Julian, R. WO2019/161162, 2019, A1 Location in patent:Paragraph 00114; 00218-00219

107018-37-1
31 suppliers
inquiry

23516-85-0
43 suppliers
inquiry
105-07-7
387 suppliers
$5.00/5g

23516-85-0
43 suppliers
inquiry