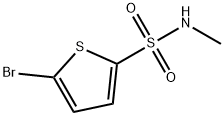
5-BroMo-thiophene-2-sulfonic acid M ethylaMide synthesis
- Product Name:5-BroMo-thiophene-2-sulfonic acid M ethylaMide
- CAS Number:81597-52-6
- Molecular formula:C5H6BrNO2S2
- Molecular Weight:256.14
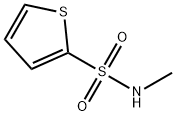
53442-30-1
21 suppliers
$45.00/25mg

81597-52-6
21 suppliers
$181.25/1g
Yield:81597-52-6 19%
Reaction Conditions:
with bromine in chloroform; for 7 h;Reflux;
Steps:
26.b
b) 5-Bromo-/V-methylthiophene-2-sulfonamideBr2 (3 ml_, 51.46 mmol) was added to a solution of λ/-methylthiophene-2- sulfonamide (4.56 g, 25.73 mmol) in CHCI3 (70 ml_). The reaction mixture was refluxed for 7 h, allowed to reach r.t., poured into H2O (100 ml_) and extracted with CH2CI2 (100 ml_). The organic layer was dried over Na2SO4 (anhydrous), filtered and concentrated. The crude residue was flash chromatographed on SiO2 (15→30% EtOAc/hexanes) to furnish 1.23 g of 5-bromo-/V- methylthiophene-2-sulfonamide (off-white solid, yield: 19%). 1H NMR (CDCI3, 250 MHz) δ ppm: 7.61 (m, 1 H), 7.10 (dd, J = 3.8 Hz, 4.5 Hz, 1 H), 4.79 (bs, 1 H), 2.73 (d, J = 5.2 Hz, 3H).
References:
WO2009/80722,2009,A2 Location in patent:Page/Page column 45
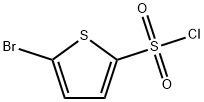
55854-46-1
235 suppliers
$6.00/1g

74-89-5
7 suppliers
$13.44/25ML

81597-52-6
21 suppliers
$181.25/1g

55854-46-1
235 suppliers
$6.00/1g
593-51-1
532 suppliers
$21.00/100g

81597-52-6
21 suppliers
$181.25/1g

![1-[2-(1-Piperidyl)phenyl]ethanol](/CAS/20180703/GIF/78648-37-0.gif)