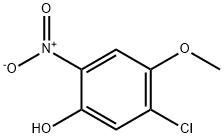
5-Chloro-4-methoxy-2-nitro-phenol synthesis
- Product Name:5-Chloro-4-methoxy-2-nitro-phenol
- CAS Number:14164-14-8
- Molecular formula:C7H6ClNO4
- Molecular Weight:203.58
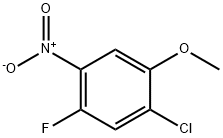
84478-76-2
122 suppliers
$5.00/100mg

14164-14-8
9 suppliers
$533.00/5g
Yield:14164-14-8 90%
Reaction Conditions:
with sodium hydroxide in water at 90; for 20 h;
Steps:
5-Chloro-4-methoxy-2-nitrophenol
To a solution of 1 -chloro-5-fluoro-2-methoxy-4-nitrobenzene (4.Og, 19. 5mmol) in H20 (50 mL) was added NaOH (8.0 g, 195 mmol) at rt. The reaction mixture was stirred at 90 00 for 20h. TLC showed the reaction to be complete. The reaction mixture was poured into ice-water (lOOmL), acidified to pH 2 with 1.0 N HCI and extracted with EtOAc (3x5OmL). The organic layer was washed with brine (lOOmL), dried (Na2SO4), filtered and concentrated under reduced pressure to give give 5- chloro-4-methoxy-2-nitrophenol as a yellow solid. Yield: 3.6g (90%); 1H NMR (400 MHz, DMSO-d6): 10.85 (bs, 1H), 7.60 (5, 1H), 7.24 (5, 1H), 3.85 (5, 3H); MS (ESI-) for CHNOS m/z202.11 [M-H].
References:
WO2018/37223,2018,A1 Location in patent:Page/Page column 206; 207
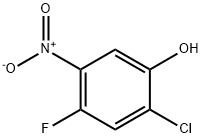
84478-75-1
193 suppliers
$10.00/1g

14164-14-8
9 suppliers
$533.00/5g