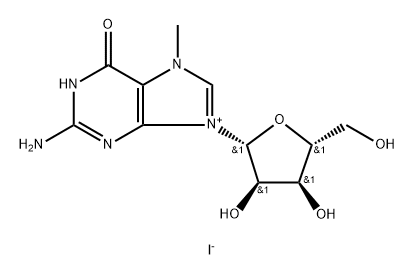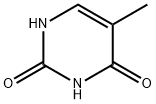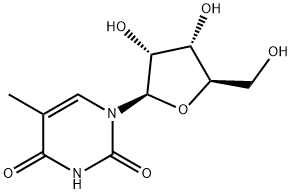
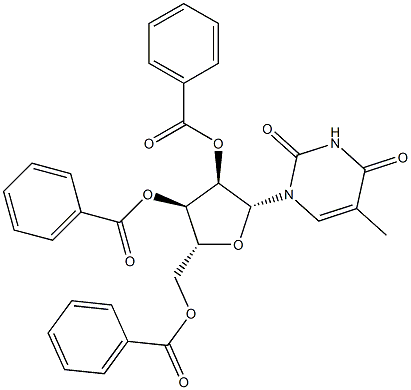
5-Methyluridine synthesis
- Product Name:5-Methyluridine
- CAS Number:1463-10-1
- Molecular formula:C10H14N2O6
- Molecular Weight:258.23
3180-76-5
1 suppliers
inquiry
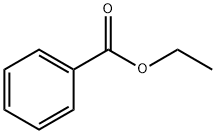
93-89-0
504 suppliers
$13.00/100g
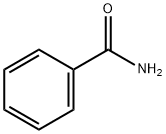
55-21-0
387 suppliers
$5.00/5G

1463-10-1
371 suppliers
$5.00/250mg
Yield:1463-10-1 95%
Reaction Conditions:
with ammonia in ethanol at 20;
Steps:
6 Example 6; 1-(β-L-Ribofuranosyl)thymine (4, B=thymine)
Compound 3 (B=thymine, R2=Bz) prepared above (28 g, 0.05 mol) in 560 mL of ethanolic ammonia (saturated at 0° C.) is kept standing overnight at room temperature. The solution is concentrated in vacuo, and the residue is triturated with diethyl ether (200 mL×2) to remove ethyl benzoate and benzamide. The insoluble residue is crystallized from ethanol to give 13.0 g (95%) of 4 (B=thymine), mp 183-185° C. The 1H-NMR spectrum of this sample is identical to that of the D-counterpart. (Fox, J. J.; Yung, N.; Davoll, J.; Brown, G. B. J. Am. Chem. Soc. 1956, 78, 2117.)
References:
US2005/90660,2005,A1 Location in patent:Page/Page column 36
![Uridine, 5'-O-[(1,1-dimethylethyl)dimethylsilyl]-5-methyl-](/CAS/20210305/GIF/119794-52-4.gif)
119794-52-4
0 suppliers
inquiry

1463-10-1
371 suppliers
$5.00/250mg
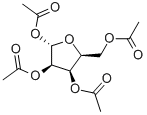
144490-03-9
123 suppliers
$30.00/1g
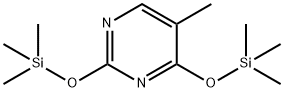
7288-28-0
52 suppliers
$60.00/250mg

1463-10-1
371 suppliers
$5.00/250mg
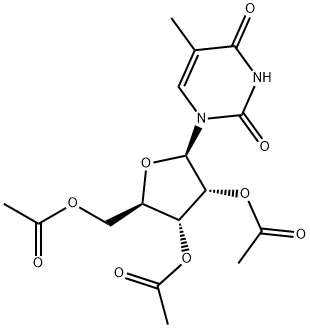
4336-39-4
0 suppliers
inquiry

1463-10-1
371 suppliers
$5.00/250mg