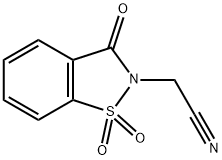
(1,1,3-TRIOXO-1,3-DIHYDRO-1LAMBDA6-BENZO[D]ISOTHIAZOL-2-YL)-ACETONITRILE synthesis
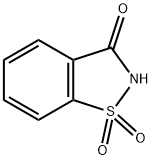
- Product Name:(1,1,3-TRIOXO-1,3-DIHYDRO-1LAMBDA6-BENZO[D]ISOTHIAZOL-2-YL)-ACETONITRILE
- CAS Number:52188-12-2
- Molecular formula:C9H6N2O3S
- Molecular Weight:222.22
Yield:52188-12-2 82%
Reaction Conditions:
with potassium carbonate;potassium iodide in N,N-dimethyl-formamide at 80; for 48 h;
Steps:
5.2.1. 2-(1,1-Dioxido-3-oxobenzo[d]isothiazol-2(3H)-yl)acetonitrile (1)
1.66g of potassium carbonate and 0.18g of potassium iodide (10mol%) were added to a stirring solution of saccharin (2.0g, 1.0equiv) in 10mL of N,N-dimethylformamide. 2.8mL of chloroacetonitrile (4.0equiv) were added portionwise and the reaction stirred at 80°C for 48h. The reaction was poured on ice and the resulting suspension was filtered to give title compound as a light brown solid (2.0g, 82% yield); mp 130-134°C; IR νmax 3104 (ν Csp2-H), 2260 (ν CN), 1744 (ν C=O), 1336 (νas S=O), 1251 (ν C-N), 1165 (νs S=O), 728 (δ Csp2-H), 676 (δ Csp2-H) cm-1; 1H NMR (400MHz, DMSO-d6) δ 5.11 (2H, s, CH2), 8.05 (1H, t, J=7.6Hz, CHAr), 8.12 (1H, t, J=7.6Hz, CHAr), 8.19 (1H, d, J=7.6Hz, CHAr), 8.40 (1H, d, J=7.6Hz, CHAr). 13C NMR (101MHz, DMSO-d6) δ 25.9 (CH2), 115.35 (CN), 122.5 (Ar), 126.1 (Ar), 126.2 (Ar), 136.1 (Ar), 136.9 (Ar), 137.2 (Ar), 158.4 (C=O).
References:
D'Ascenzio, Melissa;Carradori, Simone;De Monte, Celeste;Secci, Daniela;Ceruso, Mariangela;Supuran, Claudiu T. [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 6,p. 1821 - 1831]