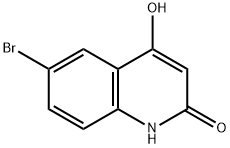
6-broMo-4-hydroxyquinolin-2(1H)-one synthesis
- Product Name:6-broMo-4-hydroxyquinolin-2(1H)-one
- CAS Number:54675-23-9
- Molecular formula:C9H6BrNO2
- Molecular Weight:240.05
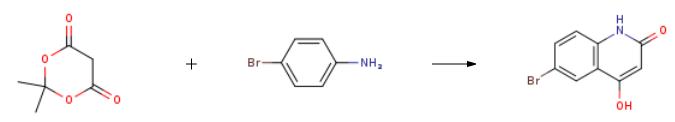
Literature [Synthetic Communications 2010, 40, 732] in accordance with the general method described, 4-bromoaniline (30.0 g, 174 mmol) and 2,2-dimethyl-l, 3-dioxane-4,6-dione ( 25.1 g, was heated in 174 mmol) in 80 for 1.5 hours, cooled to ambient temperature, 3 - ((4-bromophenyl) amino) -3-oxo-propane to give the acid.By removing the acetone by-product under vacuum to give the intermediate product as a dry solid.It was added to Eaton's Reagent (100 mL) to the solid, the mixture obtained in and then heated to 70 overnight, cooled to room temperature.The mixture was poured into water, the brown precipitate was filtered, and rinsed with water.With ethanol, the brown precipitate tree illustration in the tube, and then filtered to give the title compound as a light brown solid.
95262-09-2
26 suppliers
$24.00/100mg

54675-23-9
88 suppliers
$9.00/100mg
Yield:54675-23-9 100%
Reaction Conditions:
with PPA;Polyphosphoric acid (PPA) at 120; for 1 h;
Steps:
7.B
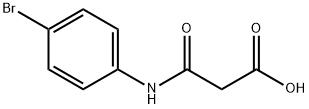
7B. 6-Bromo-4-hydroxy-lH-qumolin-2-one: To 7A (2.8g, 9.8 mmol) in methanol/water (30 mL/10 mL) was added sodium carbonate (1.55g, 14.6 mmol) and the mixture was stirred at rt for 3 days. The reaction mixture was added slowly to a stirred solution of aq. 1.0 N HCl (150 mL). The resulting white precipitate was collected by filtration. The solid cake was washed thoroughly with water and then dried under vacuum to give 2.57g (100%) of the acid. MS 259.9 (M+2+H)+. A mixture of the acid (0.7Og, 2.7mmol) and PPA (ca. 10 g) was stirred at 1200C under argon. After ca. 1 h, the reaction was cooled to rt and carefully poured onto ice. The resulting white precipitate was collected and washed thoroughly with water to give 0.65g (100%) of 7B. MS 241.91 (M+2+H)+. 1H NMR (400 MHz, DMSO-d6) δ: 5.75 (s, 1 H) 7.21 (d, J=8.79 Hz, 1 H) 7.65 (dd, J=8.79, 2.20 Hz, 1 H)7.85 (d, /=2.20 Hz, 1 H).
References:
WO2007/70818,2007,A1 Location in patent:Page/Page column 101-102

106-40-1
456 suppliers
$12.00/5g

105-53-3
729 suppliers
$5.00/25g

54675-23-9
88 suppliers
$9.00/100mg

106-40-1
456 suppliers
$12.00/5g

54675-23-9
88 suppliers
$9.00/100mg
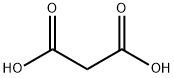
141-82-2
726 suppliers
$5.00/25g

106-40-1
456 suppliers
$12.00/5g

54675-23-9
88 suppliers
$9.00/100mg