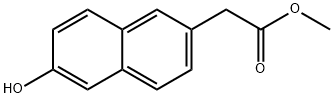
6-Hydroxy-2-naphthaleneacetic Acid Methyl Ester synthesis
- Product Name:6-Hydroxy-2-naphthaleneacetic Acid Methyl Ester
- CAS Number:91903-08-1
- Molecular formula:C13H12O3
- Molecular Weight:216.23

67-56-1
731 suppliers
$9.00/25ml
10441-46-0
30 suppliers
$85.00/50mg

91903-08-1
28 suppliers
inquiry
Yield:91903-08-1 80%
Reaction Conditions:
with sulfuric acid for 16 h;Reflux;
Steps:
106.1 Example 106: 9-(2-(5-(trifluoromethyl)-6-((cis-4-(trifluoromethyl)cyclohexyl)oxy)naphthalene-2-yl) acetyl)-9-azabicyclo[3.3.1]nonane-3-carboxylic acid Step 1: methyl 2-(6-hvdroxynaphthalen-2-yl)acetate
Example 106: 9-(2-(5-(trifluoromethyl)-6-((cis-4-(trifluoromethyl)cyclohexyl)oxy)naphthalene-2-yl) acetyl)-9-azabicyclo[3.3.1]nonane-3-carboxylic acid Step 1: methyl 2-(6-hvdroxynaphthalen-2-yl)acetate To a solution of 2-(6-hydroxynaphthalen-2-yl)acetic acid (830 mg, 4.1 mmol) in 20 mL of MeOH was added 4 drops of cone. H2SO4. The reaction mixture was heated at reflux for 16 h and concentrated. The residue was purified by column chromatography on silica gel (petroleum ether/EtOAc = 4/1) to give the title compound as a white solid (710 mg, yield 80%). 1H NMR (400 MHz, DMSO-d6) δ 9.70 (s, 1H), 7.70 (d, / = 8.8 Hz, 1H), 7.64-7.62 (m, 2H), 7.28 (dd, / = 2.0 Hz, 8.8 Hz, 1H), 7.09-7.05 (m, 2H), 3.77 (s, 2H), 3.62 (s, 3H); ESI-MS (M+H) +: 216.9. Step 2: methyl 2-(6-((c -4-(trifluoromethyl)cvclohexyl)oxy)naphthalene-2-yl)acetate A mixture of methyl 2-(6-hydroxynaphthalen-2-yl)acetate (710 mg, 3.28 mmol), iraras-4-(trifluoromethyl)cyclohexyl methanesulfonate (1.2 g, 4.93 mmol, 1.5 eq) and CS2CO3 (1.6 g, 4.93 mmol, 1.5 eq) in 10 mL of i-BuOH was heated at reflux for 16 h and cooled down. The mixture was poured into 50 mL of water and extracted with EtOAc (30 mLx3). The combined organics were dried and concentrated to give a dark brown solid (560 mg, yield 50%), ESI-MS (M+H) +: 353.1. The crude was then refluxed in methanol with concentrated H2S04 to convert the acid to corresponding methyl ester. Worked up and purified on silica gel column to give the title compound as a white solid (535 mg, yield: 91%.) 1H NMR (400 MHz, CDC13) δ 7.71 (d, / = 8.8 Hz, 1H), 7.68-7.65 (m, 2H), 7.36 (dd, J = 1.6 Hz, 8.4 Hz, 1H), 7.18-7.14 (m, 2H), 4.73-4.71 (m, 1H), 3.75 (s, 2H), 3.70 (s, 3H), 2.26-2.22 (m, 2H), 2.13-2.09 (m, 1H), 1.84-1.74 (m, 4H), 1.62-1.58 (m, 2H); ESI-MS (M+H) +: 367.1. Step 3: methyl 2-(5-iodo-6-((cis-4-(trifluoromethyl)cyclohexyl)oxy)naphthalen-2-yl)acetate To a solution of methyl 2-(6-((cis-4-(trifluoromethyl)cyclohexyl)oxy)naphthalene-2-yl)acetate (535 mg, 1.46 mmol) in MeCN (5 mL) was added NIS (361 mg, 1.60 mmol, 1.1 eq), followed by TFA (50 mg, 0.44 mmol, 0.3 eq). The mixture was stirred at rt for 16 h. The reaction mixture was diluted with water (50 mL) and extracted with EtOAc (50 mLx3). The combined organic layers were dried and concentrated. The crude product was purified by column chromatography on silica gel (petroleum ether/EtOAc = 20/1) to give the title compound as a white solid (500 g, yield: 70%). 1H NMR (400 MHz, CDC13) δ 8.10 (d, / = 8.4 Hz, 1H), 7.74 (d, /= 8.8 Hz, 1H), 7.63 (s, 1H), 7.45 (dd, /= 1.6 Hz, 8.8 Hz, 1H), 7.14 (d, /= 8.8 Hz, 1H), 4.85 (s, 1H), 3.98 (s, 2H), 3.78 (s, 3H), 2.22-2.05 (m, 5H), 1.79-1.76 (m, 2H), 1.61-1.55 (m, 2H). Step 4: 2-(5-(trifluoromethyl)-6-((cis-4-(trifluoromethyl)cyclohexyl)oxy)naphthalen-2-yl)acetic acid The title compound was prepared according to the procedure for 6-((cis-4-Methylcyclohexyl)oxy)-5-(trifluoromethyl)-2-naphthoic acid from methyl 2-(5-iodo-6-((cis-4-(trifluoromethyl)cyclohexyl)oxy)naphthalen-2-yl)acetate. 1H NMR (400 MHz, CD3OD) δ 8.12 (d, / = 8.8 Hz, 1H), 8.03 (d, / = 9.2 Hz, 1H), 7.77 (s, 1H), 7.53-7.43 (m, 2H), 4.92 (s, 1H), 3.76 (s, 2H), 2.26-2.24 (m, 1H), 2.18-1.24 (m, 2H), 1.88-1.73 (m, 6H); ESI-MS (M+H) +: 421.1. Step 5: methyl 9-(2-(5-(trifluoromethyl)-6-((cis-4-(trifluoromethyl)cvclohexyl)oxy)naphthalene-2-yl)acet yl)-9-azabicyclor3.3.11nonane-3-carboxylate To a solution of 2-(5-(trifluoromethyl)-6-((cis-4-(trifluoromethyl)cyclohexyl)oxy)naphthalen-2-yl)acetic acid (200 mg, 0.476 mmol, 1.0 eq) and methyl 9-azabicyclo[3.3.1]nonane-3-carboxylate, HCl salt (96 mg, 0.524 mmol, 1.1 eq) in CH2C12 (10 mL) was added HATU (362 mg, 0.952 mmol, 2.0 eq), followed by TEA (96 mg, 0.952 mmol, 2.0 eq). The mixture was stirred at rt for 5 h and diluted with 20 mL of water. The mixture was extracted with DCM (20 mLx3) and the combined organics were dried and concentrated. The residue was purified by column chromatography on silica gel (petroleum ether/EtOAc = 4:1) to give the title compound as a colorless oil (220 mg, yield: 80%). 1H NMR (400 MHz, CDC13) δ 8.11 (d, / = 8.4 Hz, 1H), 7.82 (d, / = 9.2 Hz, 1H), 7.61 (s, 1H), 7.38 (dd, / = 1.6 Hz, 8.8 Hz, 1H), 7.19-7.17 (m, 1 H), 4.87 (s, 1H), 4.75-4.73 (m, 1H), 4.08-4.06 (m, 1H), 3.76 (s, 2H), 3.57 (s, 3H), 3.19-3.15 (m, 1H), 2.16-2.00 (m, 3H), 1.90-1.50 (m, 16H); ESI-MS (M+H) +: 586.2. Step 6: 9-(2-(5-(trifluoromethyl)-6-((cis-4-(trifluoromethyl)cvclohexyl)oxy)naphthalene-2-yl)acet yl)-9-azabicvclor3.3.11nonane-3-carboxylic acid The title compound was prepared according to the procedure for Example 104 as a white solid (15 mg, yield: 23%). 1H NMR (400 MHz, CD3OD) δ: 8.14 (d, / = 8.4 Hz, 1H), 8.03 (d, / = 9.2 Hz, 1H), 7.78 (s, 1H), 7.53-7.48 (m, 2H), 4.98 (s, 1H), 4.85-4.82 (m, 1H), 4.35-4.32 (m, 1H), 3.95-3.92 (m, 2H), 3.30-3.25 (m, 1H), 2.29-2.16 (m, 3H), 2.04-1.54 (m, 16H); ESI-MS (M+H) +: 572.3.
References:
WO2014/18891,2014,A1 Location in patent:Page/Page column 188; 189

23981-48-8
29 suppliers
inquiry

91903-08-1
28 suppliers
inquiry
3900-45-6
381 suppliers
$10.00/10g

91903-08-1
28 suppliers
inquiry