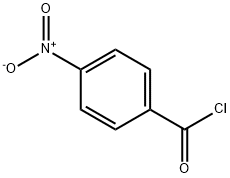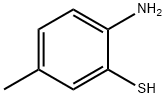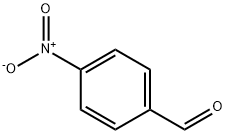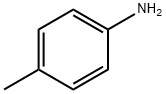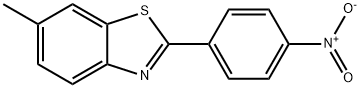
6-Methyl-2-(4-nitrophenyl)benzothiazole synthesis
- Product Name:6-Methyl-2-(4-nitrophenyl)benzothiazole
- CAS Number:488722-57-2
- Molecular formula:C14H10N2O2S
- Molecular Weight:270.31
Yield:488722-57-2 95%
Reaction Conditions:
in neat (no solvent); for 0.5 h;Green chemistry;Time;
Steps:
General procedure for the synthesis of benzo[d]thiazoles:
General procedure: A mixture of 2-aminobenzenethiol (0.2 g, 1.6 mmol) and corresponding acyl chlorides was stirred at ambient temperature or 35 °C with stirring under water (10 mL) or solvent free condition for 1-7 h. After stirring 30 min the whole reaction mixture melts, in the case of the water as solvent turned to a homogeneous liquid and in solvent free reaction forms a paste. The progress of the reaction was monitored by thin layer chromatography and complete transformation was observed in a short period of 1-7 h. The reaction mixture was then poured into methylene chloride (30 mL), and separated only organic layer. The organic layer washed with water (2 × 20 mL), dried over MgSO4 and evaporation of organic layer gave crude product. The crude product was purified by column chromatography gave pure liquid or solid product.
References:
Yoon, Soon Byung;Chun, Eun Jeong;Noh, Young Ri;Yoon, Yong Jin;Lee, Sang-Geyong [Bulletin of the Korean Chemical Society,2013,vol. 34,# 9,p. 2819 - 2821]
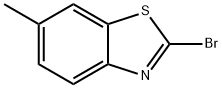
3622-19-3
63 suppliers
$64.00/100mg

171364-83-3
177 suppliers
$7.00/250mg

488722-57-2
4 suppliers
inquiry

571178-53-5
1 suppliers
inquiry

488722-57-2
4 suppliers
inquiry