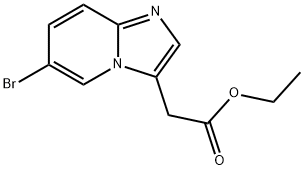
ethyl 2-(6-bromoH-imidazo[1,2-a]pyridin-3-yl)acetate synthesis
- Product Name:ethyl 2-(6-bromoH-imidazo[1,2-a]pyridin-3-yl)acetate
- CAS Number:603311-76-8
- Molecular formula:C11H11BrN2O2
- Molecular Weight:283.12
1072-97-5
697 suppliers
$9.00/1g

19255-60-8
1 suppliers
inquiry
![ethyl 2-(6-bromoH-imidazo[1,2-a]pyridin-3-yl)acetate](/CAS/GIF/603311-76-8.gif)
603311-76-8
68 suppliers
$68.00/100mg
Yield:-
Reaction Conditions:
Stage #1: 4,4-dimethoxy-but-2-enoic acid ethyl esterwith toluene-4-sulfonic acid in water;acetonitrile; for 0.5 h;Heating / reflux;
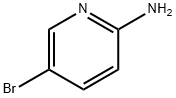
Stage #2: 5-bromo-2-pyridylamine in water;acetonitrile; for 15 h;Heating / reflux;
Steps:
10.A A. Preparation of (6-Bromo-imidazo[1,2-a]pyridin-3-yl)-acetic acid ethyl ester
Reflux a solution of 4, 4-dimethoxy-but-2-enoic acid ethyl ester (3.3 g, 19.12 mmol) and toluene-4-sulfonic acid (0.02 g, 0.12 mmol) in a solution of 2: 1 acetonitrile: water (90 mL) for 30 min. Add 2-AMINO-5-BROMO-PYRIDINE (1.65 g, 9.56 mmol) and reflux for 15 h. Cool and concentrate TO-20 ML. Dilute the reaction with saturated aqueous sodium bicarbonate and extract into ethyl acetate. Combine and concentrate organic extracts. Flash chromatography using ethyl acetate/dichloromethane/methanol mixtures gives the subtitled compound as a brown oil (2. 9 G). TOF MS ES+ exact mass calculated for CLLHNBRN202 (P+1) : m/z = 283.0075 Found: 283. 0082. 1H NMR (400 MHz, DMSO-d6) B 8.68 (s, 1H), 7.55 (d, J = 10 Hz, 1H), 7.49 (s, 1H), 7.34 (d, J = 10 Hz, 1H), 4.15 (s, 2H), 4.09 (q, J = 4 Hz, 2H), 1.18 (t, J = 4 Hz, 3H). EA Calcd. For CLLHLLBRN202 : C, 46.66 ; H, 3.92 ; N, 9.89 ; Found C, 46.56 ; H, 4.02 ; N, 9.60.
References:
WO2004/50659,2004,A1 Location in patent:Page 39