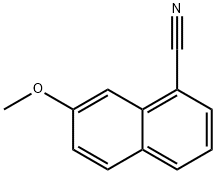
7-METHOXY-1-NAPHTHONITRILE synthesis
- Product Name:7-METHOXY-1-NAPHTHONITRILE
- CAS Number:158365-54-9
- Molecular formula:C12H9NO
- Molecular Weight:183.21
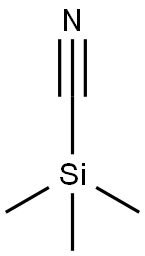
7677-24-9
366 suppliers
$19.00/5g
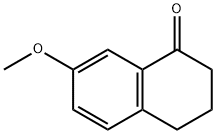
6836-19-7
485 suppliers
$14.00/5g

158365-54-9
21 suppliers
inquiry
Yield:158365-54-9 78%
Reaction Conditions:
Stage #1: trimethylsilyl cyanide;7-Methoxy-1-tetralone;zinc(II) iodide in toluene at 45; for 3.33333 h;Reflux;
Stage #2: with pyridine;trichlorophosphate in toluene at 35;Reflux;
Stage #3: with 2,3-dicyano-5,6-dichloro-p-benzoquinone in toluene; for 2 h;Reflux;
Steps:
9
7-Methoxy-1-tetralone (8.16 g, 46 mmol, 1 eq) and ZnI2 (0.365 g, 1 mmol, 0.025 eq) were dissolved in 25 ml of toluene and heated at 45° C. Trimethylsilylcyanide (TMSCN) (5.0 g, 50 mmol, 1.1 eq) is added over a period of 20 min, and all is refluxed for 3 h. After cooling the mixture at about 35° C., pyridine (5.5 ml, 69 mmol, 1.5 eq) and POCl3 (6.4 ml, 69 mmol, 1.5 eq) are added, and the mixture is boiled under reflux for another 6 h. Thereafter, 80 ml of a 3 N NaOH cooled at 3° C. is added over a period of 15 min. The aqueous phase is extracted with 48 ml of toluene, and the organic phase is washed twice with 40 ml of 1 N NaOH, once with 40 ml of water, three times with 40 ml of 3 N HCl, once with 40 ml of water, once with 40 ml of a saturated NaHCO3 solution and once with a saturated sodium chloride solution. After the addition of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) (8.41 g, 37 mmol, 0.8 eq) over 20 min, the organic phase is boiled under reflux for 2 h. After cooling, the precipitate was filtered off and washed twice with 32 ml of 1 N NaOH and once with 32 ml of a saturated sodium chloride solution. Drying of the precipitate yielded the desired product in a yield of 78%, 6.6 g. C12H9NO; MW 183; Rf value (hexane/ethyl acetate 8/2): 0.7; 1H-NMR (CDCl3): δ 7.97 (d, J=8.2 Hz, 1H), 7.85 (dd, J=0.9 Hz, J=6.9 Hz, 1H), 7.79 (d, J=9.1 Hz, 1H), 7.45 (d, J=2.2 Hz, 1H), 7.36 (dd, J=8.2 Hz, J=7.3 Hz, 1H), 7.24 (dd, J=2.5 Hz, J=8.8 Hz, 1H), 3.98 (s, 3H); IR: 3003, 2943, 2839, 2218, 1505, 1259, 1243, 1025 1/cm
References:
US2010/204234,2010,A1 Location in patent:Page/Page column 14

158365-53-8
3 suppliers
inquiry

158365-54-9
21 suppliers
inquiry

6836-19-7
485 suppliers
$14.00/5g

158365-54-9
21 suppliers
inquiry

29921-51-5
18 suppliers
inquiry

158365-54-9
21 suppliers
inquiry
118-46-7
209 suppliers
$10.00/1g

158365-54-9
21 suppliers
inquiry