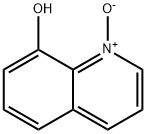
8-Hydroxyquinoline-N-oxide synthesis
- Product Name:8-Hydroxyquinoline-N-oxide
- CAS Number:1127-45-3
- Molecular formula:C9H7NO2
- Molecular Weight:161.16
Yield:-
Reaction Conditions:
in chloroform;
Steps:
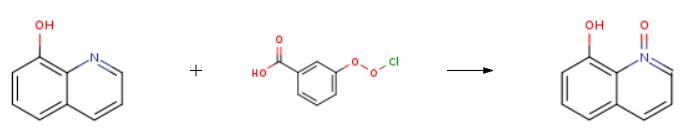
1 8-Hydroxyquinoline-N-Oxide
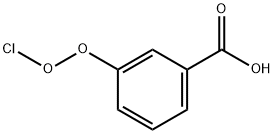
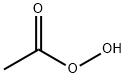
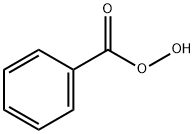
EXAMPLE 1 8-Hydroxyquinoline-N-Oxide In this example, 8-hydroxyquinoline-N-oxide was produced from 8-hydroxyquinoline. A stirred solution of 8-hydroxyquinoline (25.00 g, 172.2 mmol) in 550 ml of CHCl3 was cooled to 0° C., and 3 -chloroperoxybenzoic acid (40.00 g, 80% Tech. grade *0.231 mmol=0.185 mmol) was added slowly over 3 minutes. The solution was kept at 0° C. and stirred for 3 hours. During this period, the 3-chlorobenzoic acid byproduct precipitated. The 3-chlorobenzoic acid was removed by filtration and the orange filtrate was concentrated to dryness and the remaining solid was triturated with 2% NH40 H (2*200 ml). The solid was isolated on a frit and washed with H2 O.
References:
US5021567,1991,A
148-24-3
805 suppliers
$5.00/25g

1127-45-3
214 suppliers
$6.00/1g