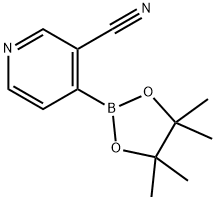
3-CYANO-4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)PYRIDINE synthesis
- Product Name:3-CYANO-4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)PYRIDINE
- CAS Number:878194-92-4
- Molecular formula:C12H15BN2O2
- Molecular Weight:230.07
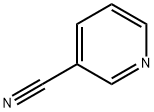
100-54-9
534 suppliers
$5.00/10g
76-09-5
373 suppliers
$8.19/250mg
![3-CYANO-4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)PYRIDINE](/CAS/GIF/878194-92-4.gif)
878194-92-4
78 suppliers
$44.00/50 mg
Yield:878194-92-4 18%
Reaction Conditions:
Stage #1:pyridine-3-carbonitrile with Triisopropyl borate;2,2,6,6-tetramethylpiperidinyl-lithium in tetrahydrofuran;hexane at -78 - 20;Inert atmosphere;
Stage #2: with acetic acid in tetrahydrofuran;hexane at 20;Inert atmosphere;
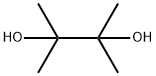
Stage #3:2,3-dimethyl-2,3-butane diol in tetrahydrofuran;hexane at 20; for 2 h;Inert atmosphere;
Steps:
4.3.1. 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)nicotinonitrile (3b)
In a dry 500 mL flask under N2 2,2,6,6-tetramethylpiperidine (57.6 mmol) was dissolved in dry THF (200 mL) and the mixture was cooled to -10 °C before n-BuLi (57.6 mmol, 2.5 M in n-hexane) was added over 2 min. The mixture was stirred for 10 min before cooling to -78 °C. At -78 °C, B(O-i-Pr)3 (65.3 mmol) was added over 2 min and stirred for 5 min at -78 °C before 3-cyanopyridine (48 mmol) dissolved in dry THF (50 mL) was added dropwise over 5 min. The reaction was left in the dry ice bath, allowed to reach room temperature overnight and quenched with glacial acetic acid (67.2 mmol) followed by addition of pinacol (72 mmol). The mixture was stirred for 2 h at room temperature and then transferred to a separating funnel with CH2Cl2 (75 mL) and washed with aqueous KH2PO4 (10 w/v %) (4×60 mL). The combined aqueous layers was back-extracted once with CH2Cl2 (15 mL), the combined organic phase was dried over MgSO4, and the solvents were evaporated to give cyanopyridineboronic ester 3b as a yellow solid with 18% yield; mp 88 °C; IR (KBr) ν 3097, 2968, 2930, 2238 (CN), 1618, 1522, 1476, 1464, 1421, 1385, 1373, 1337, 1201, 1113, 1046, 963, 882, 849, 778, 765, 714, 655, 578 cm-1; 1H NMR (400 MHz, CDCl3) δ 8.86 (s, 1H, H2), 8.74 (d, J=4.6 Hz, 1H, H6), 7.70 (d, J=4.6 Hz, 1H, H5), 1.34 (s, 12H); 13C NMR (100 MHz, CDCl3) δ 153.3, 152.1, 129.3, 117.1, 114.2, 85.8, 83.1, 25.0.
References:
Rochais, Christophe;Yougnia, Rodrigue;Cailly, Thomas;Sopková-De Oliveira Santos, Jana;Rault, Sylvain;Dallemagne, Patrick [Tetrahedron,2011,vol. 67,# 32,p. 5806 - 5810] Location in patent:experimental part
874290-89-8
56 suppliers
inquiry

76-09-5
373 suppliers
$8.19/250mg
![3-CYANO-4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)PYRIDINE](/CAS/GIF/878194-92-4.gif)
878194-92-4
78 suppliers
$44.00/50 mg

100-54-9
534 suppliers
$5.00/10g
![3-CYANO-4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)PYRIDINE](/CAS/GIF/878194-92-4.gif)
878194-92-4
78 suppliers
$44.00/50 mg