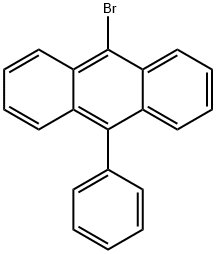
9-Bromo-10-phenylanthracene synthesis
- Product Name:9-Bromo-10-phenylanthracene
- CAS Number:23674-20-6
- Molecular formula:C20H13Br
- Molecular Weight:333.22
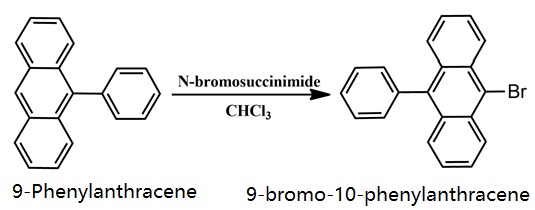
Synthesis of 9-bromo-10-phenylanthracene
602-55-1
173 suppliers
$6.00/250mg

23674-20-6
251 suppliers
$11.00/1g
Yield:23674-20-6 98%
Reaction Conditions:
with N-Bromosuccinimide in chloroform at 60; for 1 h;Inert atmosphere;
Steps:
1.2 Synthesis of 9-bromo-10-phenylanthracene (2)
9-phenylanthracene (1) (2.54 g, 10.0 mmol), N-bromosuccinimide (2.14 g, 12.0 mmol) and chloroform (200 mL) were heated at 60 ° C. for 1 hour under a nitrogen atmosphere. After cooling to room temperature, the solvent was distilled off under reduced pressure. After re-dissolving in acetone, it was reprecipitated in methanol and filtered to obtain a yellow solid (3.26 g, yield 98%).
References:
JP6381201,2018,B2 Location in patent:Paragraph 0062; 0064

359435-47-5
5 suppliers
inquiry
24388-23-6
209 suppliers
$6.00/5g

23674-20-6
251 suppliers
$11.00/1g

42908-58-7
0 suppliers
inquiry

23674-20-6
251 suppliers
$11.00/1g

98-80-6
721 suppliers
$5.00/5g

23674-20-6
251 suppliers
$11.00/1g