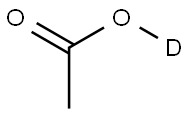
ACETIC ACID-D synthesis
- Product Name:ACETIC ACID-D
- CAS Number:758-12-3
- Molecular formula:C2H3DO2
- Molecular Weight:61.06

141-78-6
602 suppliers
$20.50/250ml

758-12-3
66 suppliers
$77.40/50g
Yield:-
Reaction Conditions:
with 3NH4(1+)*PW12O40(3-)*6H2O=(NH4)3PW12O40*6H2O;water-d2 at 59.84; for 2 h;Catalytic behavior;Reagent/catalyst;
Steps:
2.5 Hydrolysis of alkyl acetate
Hydrolysis of ethyl acetate was carried out at 333K with 5wt% ethyl acetate in D2O (total volume: 3.0ml, ethyl acetate: 0.15g (1.7mmol)) for 2h. The weight of the catalyst used was 0.075g (24.7μmol) [10]. (0016) Conversion and yield were estimated using 1H NMR. In the 1H NMR spectra, peaks corresponding to ethyl acetate, ethyl alcohol, and acetic acid were observed. No other peak was observed. Therefore, we assumed that selectivity of this hydrolysis was 100%. Since peaks of methylene (CH2O) for ethyl acetate (4.03ppm) and ethyl alcohol (3.52ppm) were well separated, the conversion of ethyl acetate was calculated using the integration ratio of these two peaks as follows:Conversion (%)=(integration of CH2O of ethyl alcohol)/(integration of CH2O of ethyl acetate+integration of CH2O of ethyl alcohol)×100. (0017) The calculated conversion and yield of hydrolysis of ethyl acetate in the presence of Keggin-type phosphotungstic acid were consistent with data obtained by the GC method, indicating that our analytical method using NMR is appropriate.
References:
Sahiro, Koichi;Ide, Yusuke;Sano, Tsuneji;Sadakane, Masahiro [Materials Research Bulletin,2013,vol. 48,# 10,p. 4157 - 4162]

64-19-7
1531 suppliers
$10.00/25ML

758-12-3
66 suppliers
$77.40/50g

75-36-5
560 suppliers
$17.92/100G

758-12-3
66 suppliers
$77.40/50g
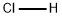
7698-05-7
84 suppliers
$22.00/10ml

75-36-5
560 suppliers
$17.92/100G

758-12-3
66 suppliers
$77.40/50g
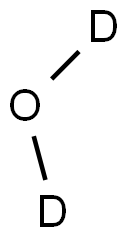
7789-20-0
172 suppliers
$18.00/25g

108-24-7
5 suppliers
$14.00/250ML

758-12-3
66 suppliers
$77.40/50g