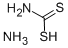
Ammonium dithiocarbamate synthesis
- Product Name:Ammonium dithiocarbamate
- CAS Number:513-74-6
- Molecular formula:CH6N2S2
- Molecular Weight:110.2
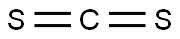
75-15-0
233 suppliers
$40.00/100 g
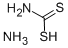
513-74-6
154 suppliers
$5.00/1g
Yield:513-74-6 96%
Reaction Conditions:
with ammonia in tetrahydrofuran at 20;
Steps:
Dimethyl carbonimidodithioate, 4.
General procedure: Following literature procedures,1 ammonia gas was bubbled into carbon disulfide 1 (16.7g, 0.22 mol) in THF (100 ml) keeping the temperature below 45 °C. After 1 hr, the reaction was stopped by allowing the temperature to decrease to rt. The excess ammonia gas was removed by streaming N2 for 30 min or by rotary evaporation under vacuum. The precipitate was collected by filtration to afford white solid ammonium dithiocarbamate 2 (23.1 g, 96%). Methyl iodide (17.8 g, 0.125 mol) was added to a solution of 2 (5.5 g, 0.05 mol) in acetone (100 ml) at rt and the mixture was stirred for 24 hr. The solid was collected by filtration, washed with chilled acetone (20 ml) and dried under vacuum to provide white solid S,S’-dimethyliminodithiocarbonate hydroiodide ammonium iodide 3 (18.9 g, 96%). 3 (7.9 g, 0.02 mol) was treated with sat. aq. NaHCO3 solution (100 ml x 2) and the organic products extracted with dichloromethane (50 ml). The organic layer was dried over Na2SO4 and concentrated in vacuo to a clear, colorless liquid 4 (2.3 g, 95%). 1H NMR (400 MHz, CDCl3) δ 8.88 (s, 1H), 2.42 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 172.10, 14.50. HRMS (ESI+) m/z calcd for C3H8N+ 122.0098, found 122.0096.
References:
Pollock, Julie A.;Kim, Sung Hoon;Katzenellenbogen, John A. [Tetrahedron Letters,2015,vol. 56,# 44,p. 6097 - 6099] Location in patent:supporting information
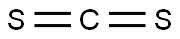
75-15-0
233 suppliers
$40.00/100 g
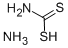
513-74-6
154 suppliers
$5.00/1g