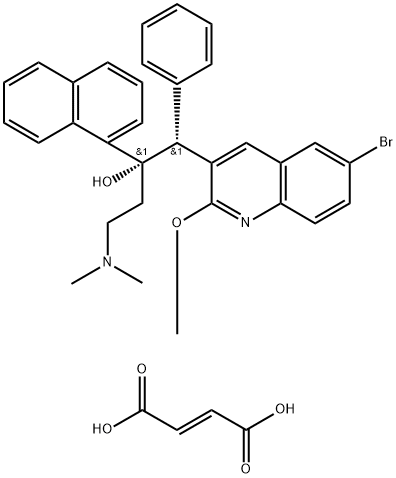
Bedaquiline fumarate synthesis
- Product Name: Bedaquiline fumarate
- CAS Number:845533-86-0
- Molecular formula:C36H35BrN2O6
- Molecular Weight:671.59
843663-66-1
215 suppliers
$40.00/2mg
110-17-8
1004 suppliers
$9.00/5g

845533-86-0
148 suppliers
inquiry
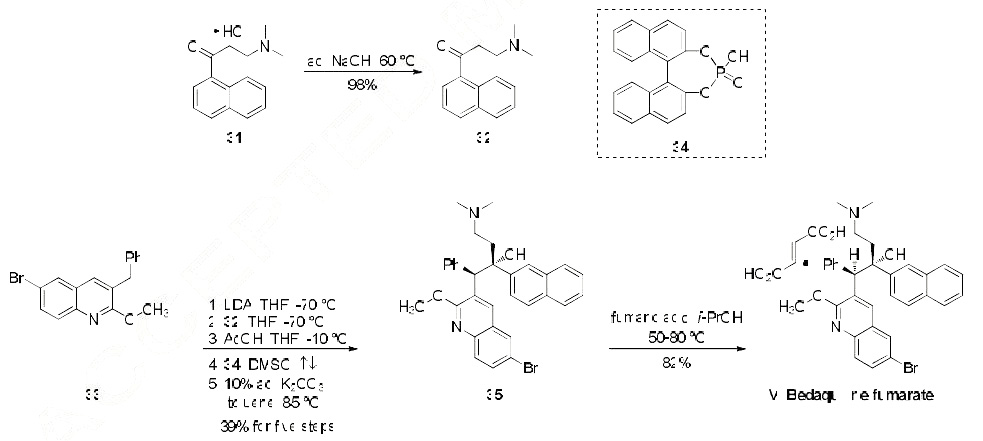
Yield:845533-86-0 82%
Reaction Conditions:
in isopropyl alcohol at 20 - 70; for 18 h;Heating / reflux;
Steps:
A
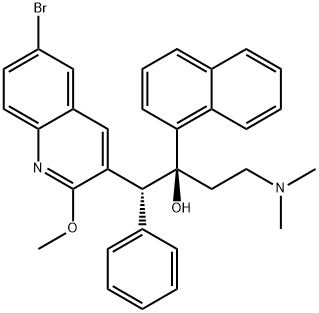
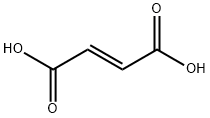
A. Synthesis of the fumarate salt of αS, βR)-6-bromo-α-[2-fdimethylamino)ethyll- 2-methoxy-α- 1-naphthalenyl- β-phenyl-3-q uinolineethanol; 1Og (0.018 mol) of (αS, βR)-6-bromo-α-[2-(dimethylamino)ethyl]-2-methoxy-α-l- naphthalenyl-β-phenyl-3-quinolineethanol and 2.13 g (0.018 mol) of fumaric acid were suspended in 185 ml isopropanol. Dicalite (0.25g) and charcoal (0.25g) were added to the suspension. The mixture was refluxed for an hour, the reaction mixture was cooled to 700C and filtered in the heat. The filter cake was washed with 10ml isopropanol.The mother liquor was slowly cooled to 500C and stirred for 1 hour at this temperature.The reaction mixture was further cooled to room temperature and stirred for 16 hours. The crystals were filtered off and washed with 20 ml isopropanol. The wet cake was dried at 500C during 16 hours.Yield: 1O g of (alpha S, beta R)-6-bromo-alpha-[2-(dimethylamino)ethyl]-2-methoxy- alpha-l-naphthalenyl-beta-phenyl-3-quinolineethanol (2E)-2-butenedioate (1 :1) (white solid) (82%).
References:
WO2008/68231,2008,A1 Location in patent:Page/Page column 16
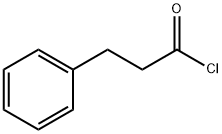
645-45-4
328 suppliers
$15.00/5g

845533-86-0
148 suppliers
inquiry
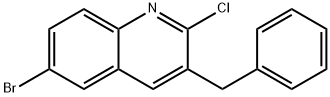
654655-68-2
112 suppliers
$5.00/250mg

845533-86-0
148 suppliers
inquiry
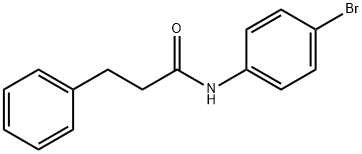
316146-27-7
89 suppliers
$32.00/5g

845533-86-0
148 suppliers
inquiry
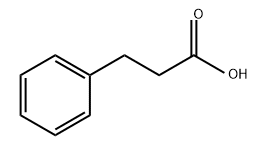
501-52-0
478 suppliers
$14.14/1gm:

845533-86-0
148 suppliers
inquiry