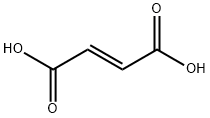
Fumaric acid
- CAS No.
- 110-17-8
- Chemical Name:
- Fumaric acid
- Synonyms
- FA;fumaric;2-BUTENEDIOIC ACID;Butenedioic acid;ACIDUM FUMARICUM;(E)-2-Butenedioic acid;TRANS-2-BUTEN-1,4-DIOIC ACID;Fumaric Acid (Food and Technical);bjss;U-1149
- CBNumber:
- CB5852804
- Molecular Formula:
- C4H4O4
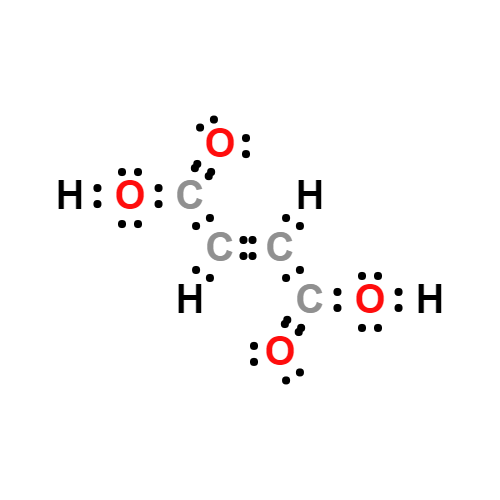
Lewis structure
- Molecular Weight:
- 116.07
- MDL Number:
- MFCD00002700
- MOL File:
- 110-17-8.mol
- MSDS File:
- SDS
| Melting point | 298-300 °C (subl.) (lit.) |
|---|---|
| Boiling point | 137.07°C (rough estimate) |
| Density | 1.62 |
| vapor pressure | 1.7 mm Hg ( 165 °C) |
| refractive index | 1.5260 (estimate) |
| FEMA | 2488 | FUMARIC ACID |
| Flash point | 230 °C |
| storage temp. | Store below +30°C. |
| solubility | 95% ethanol: soluble0.46g/10 mL, clear, colorless |
| form | Fine Crystalline Powder |
| pka | 3.02, 4.38(at 25℃) |
| color | White |
| PH | 3.19(1 mM solution);2.57(10 mM solution);2.03(100 mM solution); |
| Odor | odorless |
| Odor Type | odorless |
| explosive limit | 40% |
| Water Solubility | 0.63 g/100 mL (25 ºC) |
| Merck | 14,4287 |
| JECFA Number | 618 |
| BRN | 605763 |
| Stability | Stable at room temperature. Decomposes at around 230 C. Incompatible with strong oxidizing agents, bases, reducing agents. Combustible. |
| InChIKey | VZCYOOQTPOCHFL-OWOJBTEDSA-N |
| LogP | -4.02 at 20℃ |
| FDA 21 CFR | 172.350; 175.105; 175.300; 175.320; 176.180; 177.1200 |
| Indirect Additives used in Food Contact Substances | (E)-2-BUTENEDIOIC ACID |
| Substances Added to Food (formerly EAFUS) | FUMARIC ACID |
| CAS DataBase Reference | 110-17-8(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 88XHZ13131 |
| ATC code | D05AX01 |
| NIST Chemistry Reference | Fumaric acid(110-17-8) |
| EPA Substance Registry System | Fumaric acid (110-17-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319 | |||||||||
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36 | |||||||||
| Safety Statements | 26 | |||||||||
| RIDADR | UN 9126 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | LS9625000 | |||||||||
| Autoignition Temperature | 375 °C | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29171900 | |||||||||
| Toxicity | LD50 orally in Rabbit: 9300 mg/kg LD50 dermal Rabbit 20000 mg/kg | |||||||||
| NFPA 704 |
|
Fumaric acid price More Price(43)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W248835 | Fumaric Acid natural (US), ≥98%, FG | 110-17-8 | 1kg | $187 | 2024-03-01 | Buy |
| Sigma-Aldrich | W248835 | Fumaric Acid natural (US), ≥98%, FG | 110-17-8 | 5kg | $654 | 2024-03-01 | Buy |
| Sigma-Aldrich | W248800 | Fumaric Acid FCC, FG | 110-17-8 | 1kg | $76.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | W248800 | Fumaric Acid FCC, FG | 110-17-8 | 25kg | $703 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.00269 | Fumaric acid for synthesis | 110-17-8 | 100g | $43.7 | 2024-03-01 | Buy |
Fumaric acid Chemical Properties,Uses,Production
Description
Fumaric acid is an important kind of organic chemical raw materials as well as the intermediate of fine chemical products. Meanwhile, it is also an important kind of derivatives of maleic anhydride, being widely used in food, coatings, resins and plasticizers. In the food industry, fumaric acid, used as souring agent, can be applied to soft drinks, western-style wine, cold drinks, fruit juice concentrate, canned fruit, pickles and ice cream. As an acidic substance used as solid beverage gas production agent, it has excellent bubble durability with delicate product organization.
Fumaric acid has been used as a food acidulant since 1946. As a food additive, it is used as an acidity regulator and can be denoted by the E number E297. Chemically it is an unsaturated dicarbonic acid and is part of the citric acid cycle.
Fumaric acid is a common food additive included in many processed foods to keep them stable and to add tartness. The substance has a more sour flavor than citric acid, another common food additive. Fumaric acid occurs naturally in fumitory, bolete mushrooms, lichen and Iceland moss. As an additive, fumaric acid is produced synthetically, mainly from malic acid from apples. Fumaric acid as an additive is regulated under the Codex Alimentarius General Standard for Food Additives (GSFA), a collection of internationally recognized standards.The U.S. Food and Drug Administration considers it safe.
Chemical Properties
White, odorless granules or crystalline powder. It is soluble in alcohol, slightly soluble in water and in ether, and very slightly soluble in chloroform.
Fumaric acid is used as a replacement for tartaric acid. It has an odorless, tart, acidic-sour flavor. It may be synthesized by the action of certain fungi (Rhizopus nigricans) on glucose; by oxidation of furfural with sodium chlorate in the presence of vanadium pentoxide.
Chemical Properties
Fumaric acid is naturally presented in Corydalis, mushrooms and fresh beef. Product precipitated from the water is monoclinic needle-like, prismatic or leaf-like white crystalline or crystalline powder. It is odorless with a special and strong sour, which is about 1.5 times that of the citric acid. It has a melting point 287 ° C, the boiling point of 290 ° C with subjecting to sublimation at temperature above 200 ° C. When being heated to 230 ° C, it will lose water and become maleic anhydride. Its co-boiling with water can produce DL-malic acid. It is soluble in ethanol, slightly soluble in water and ether, but insoluble in chloroform. The pH value of the 3% aqueous solution is 2.0 to 2.5 with a strong buffering performance, in order to maintain the pH of the aqueous solution at around 3.0. This product is non-toxic; rat-oral LD50: 8000mg/kg.
Chemical Properties
Fumaric acid is odorless with a tart, acidic-sour favor Fumaric acid is used as a replacement of tartaric acid.
Chemical Properties
Fumaric acid is a colorless to white, odorless crystalline powder. Fruity-acidic taste.
Chemical Properties
Fumaric acid occurs as white, odorless or nearly odorless, granules or as a crystalline powder that is virtually nonhygroscopic.
Occurrence
Reported found in several plants, Fumaria offcinalis L , Boletus scaber Boll and lean raw fsh
Uses
1. Fumaric acid is used for the production of unsaturated polyester resin. This kind of resin is characterized by excellent resistance to chemical corrosion as well as heat resistance; the copolymer of fumaric acid and vinyl acetate is a kind of excellent adhesive. Its copolymer with styrene copolymer is the raw material for the manufacture of glass fiber. The plasticizer of the fumaric acid is non-toxic and can be applied to the vinyl acetate latex contact with food. This product is the intermediate of pharmaceutical and optical bleaching agents and other fine chemicals. Neutralization of fumaric acid with sodium carbonate can generate sodium fumarate ([17013-01-3]), and then replaced with ferrous sulfate to get iron fumarate, being the drug Fersamal used for the treatment of small red blood cell anemic. The product, as a food additive-sourness agent, used in soft drinks, fruit sugar, jelly, ice cream with most of them used in combination with sourness agent, citric acid. The monosidum salt made from the reaction between fumaric acid and sodium hydroxide can also used as sour seasoning, also used as the intermediate of synthetic resin and mordant.
2. Fumaric acid is included in many dairy-based products. These include dairy drinks such as chocolate milk, cocoa, eggnog, condensed milk and whey protein beverages. It also may be added to clotted cream, milk and cream powders and milk and cream analogues (substitutes). Fumaric acid is added to cheese products, including processed cheese and cheese substitutes. Dairy-based desserts, such as pudding, flavored yogurt, sherbet and sorbet may include fumaric acid as well. Dairy fat spreads and blended spreads can include fumaric acid, and so can preserved eggs and egg-based desserts such as custard.
3. Some processed and packaged foods have fumaric acid added to them to help stabilize them and enhance their flavor. For example, many processed meats, such as bacon and canned meats, have added fumaric acid. Frozen seafood, smoked meats and the edible casings around sausages might also have fumaric acid added to them. Fermented, canned, dried and processed fruits and vegetables can contain the food additive as well. Rice cakes and other precooked rice foods, dried or preserved eggs, mustard, vinegar, cider, wine and other alcoholic beverages are additional examples of foods that might contain fumaric acid.
Uses
fumaric acid is used to add fragrance to products and to decrease product pH. It can also help keep the pH stable. It is generally used in cleansers. Fumaric acid is naturally occurring in plants, such as lichen and Iceland moss, and in animals. For example, the skin produces fumaric acid when exposed to light. It can also F be synthetically manufactured.
Uses
Fumaric Acid is an acidulant that is a nonhygroscopic, strong acid of poor solubility. it has a solubility of 0.63 g in 100 ml of distilled water at 25°c. it dissolves slowly in cold water, but if mixed with dioctyl sodium sulfosuccinate its solubility improves. the solubility rate also increases with smaller particle size. a quantity of 0.317 kg of can replace 0.453 kg of citric acid. it is used in dry mixes such as desserts, pie fillings, and candy. it is used in dry bever- age mixes because it is storage stable, free flowing, and nonhygro- scopic. it functions as a synergistic antioxidant with bha and bht in oiland lard-base products. in gelatin desserts, it improves the flavor stability and increases shelf life and gel strength.
Uses
Occurs in many plants. Essential to vegetable and tissue respiration. Used as an antioxidant.
Fumaric acid is used in food products as an
acidifier with an acid type of taste that blends
particularly well with caramellic or sugarsweetened products, baked products, etc.
Definition
Butenedioic Acid: Either of two isomers with the formula HCOOHC:CHCOOH. Both compounds can be regarded as derivatives of ethene in which a hydrogenatom on each carbon has been replaced by a –COOH group. The compounds show cis–trans isomerism.The trans form is fumaric acid (r.d.1.64; sublimes at 165°C) and the cisform is maleic acid (r.d. 1.59; m.p.139–140°C). Both are colourless crystalline compounds used in making synthetic resins. The cis form is rather less stable than the trans form and converts to the trans form at120°C. Unlike the trans form it can eliminate water on heating to form acyclic anhydride containing a–CO.O.CO– group (maleic anhydride).Fumaric acid is an intermediate in the Krebs cycle.
Production Methods
Commercially, fumaric acid may be prepared from glucose by the
action of fungi such as Rhizopus nigricans, as a by-product in the
manufacture of maleic and phthalic anhydrides, and by the
isomerization of maleic acid using heat or a catalyst.
On the laboratory scale, fumaric acid can be prepared by the
oxidation of furfural with sodium chlorate in the presence of
vanadium pentoxide.
Definition
ChEBI: A butenedioic acid in which the C2C double bond has E geometry. It is an intermediate metabolite in the citric acid cycle.
Preparation
By the action of certain fungi (Rhizopus nigricans) on glucose; by oxidation of furfural with sodium chlorate in the pres- ence of vanadium pentoxide.
Definition
Either of two isomers. Transbutenedioic acid (fumaric acid) is a crystalline compound found in certain plants. Cisbutenedioic acid (maleic acid) is used in the manufacture of synthetic resins. It can be converted into the trans isomer by heating at 120°C.
Biotechnological Production
Currently, fumaric acid is mainly manufactured by chemical synthesis via the
precursor maleic acid, which is produced using either benzene or n-butane via
catalytic oxidation. However, there are enzymatic and fermentative
production routes for fumaric acid. Prior to the advent of inexpensive petroleumbased
chemistry, fumaric acid was produced commercially by fermentation using
organisms of the genus Rhizopus with an annual production of 4,000 metric tons
. Product concentrations from 30 to 130 g.L-1 with yields from 0.3 to 1.0 g
of fumaric acid per gram of glucose and productivities of 0.46–2.0 g.L-1.h-1 have
been reported growing on glucose .
In recent years, new approaches using metabolic engineering have been studied.
For example, fumaric acid concentrations of 28.2 g.L-1 with a productivity of
0.448 g.L-1.h-1 have been reached in fed-batch cultivation of a genetic modified
E. coli . To achieve this result, eight modifications have been implemented.
Fumaric acid could be alternatively synthesized by an enzymatic process
starting from maleic acid as in the chemical synthesis. By whole-cell biocatalysis
of the Pseudomonas alcaligenes strain XD-1, a yield of 0.698 g of fumaric acid per
gram of maleic acid and a production rate of 6.98 g.L-1.h-1 have been reached
. The process has been optimized. The formation of the byproduct malic acid
was avoided due to an inactivation of fumarase by a heat treatment of the cells
beforehand. Finally, a yield of 0.95 g fumaric acid per gram maleic acid and a
production rate of 14.25 g.L-1.h-1 have been observed.
General Description
A colorless crystalline solid. The primary hazard is the threat to the environment. Immediate steps should be taken to limit spread to the environment. Combustible, though may be difficult to ignite. Used to make paints and plastics, in food processing and preservation, and for other uses.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
Fumaric acid is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Fumaric acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions. Partial carbonization and formation of maleic anhydride occur at 446° F (open vessel).
Health Hazard
Inhalation of dust may cause respiratory irritation. Compound is non-toxic when ingested. Prolonged contact with eyes or skin may cause irritation.
Flammability and Explosibility
Non flammable
Pharmaceutical Applications
Fumaric acid is used primarily in liquid pharmaceutical preparations
as an acidulant and flavoring agent. Fumaric acid may be
included as the acid part of effervescent tablet formulations,
although this use is limited as the compound has an extremely
low solubility in water. It is also used as a chelating agent which
exhibits synergism when used in combination with other true
antioxidants.
In the design of novel pelletized formulations manufactured by
extrusion–spheronization, fumaric acid was used to aid spheronization,
favoring the production of fine pellets. It has also been
investigated as an alternative filler to lactose in pellets.
Fumaric acid has been investigated as a lubricant for effervescent
tablets, and copolymers of fumaric acid and sebacic acid have
been investigated as bioadhesive microspheres.It has been used in
film-coated pellet formulations as an acidifying agent and also to
increase drug solubility.
Fumaric acid is also used as a food additive at concentrations up
to 3600 ppm, and as a therapeutic agent in the treatment of
psoriasis and other skin disorders.
Safety Profile
Poison by intraperitoneal route. Mildly toxic by ingestion and skin contact. A skin and eye irritant. Mutation data reported. Combustible when exposed to heat or flame; can react vigorously with oxidizing materials. When heated to decomposition it emits acrid smoke and irritating fumes.
Safety
Fumaric acid is used in oral pharmaceutical formulations and food
products, and is generally regarded as a relatively nontoxic and
nonirritant material. However, acute renal failure and other adverse
reactions have occurred following the topical and systemic
therapeutic use of fumaric acid and fumaric acid derivatives in the
treatment of psoriasis or other skin disorders. Other adverse
effects of oral therapy have included disturbances of liver function,
gastrointestinal effects, and flushing.
The WHO has stated that the establishment of an estimated
acceptable daily intake of fumaric acid or its salts was unnecessary
since it is a normal constituent of body tissues.
LD50 (mouse, IP): 0.1 g/kg
LD50 (rat, oral): 9.3 g/kg
Potential Exposure
Fumaric acid is used in production of resins, polyesters, plasticizers, and alkyl surface coatings; as a food additive; as an antioxidant in resins; to make dyes.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for atleast 15 min, occasionally lifting upper and lower lids.Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and washimmediately with soap and water. Seek medical attentionimmediately. If this chemical has been inhaled, removefrom exposure, begin rescue breathing (using universalprecautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make anunconscious person vomit.
Carcinogenicity
No evidence of carcinogenicity
was found in several chronic studies with rats in
which fumaric acid was added to the diet at concentrations
up to 1.5%. As for dermal application, Swiss
mice were treated topically twice weekly with a 1% solution in acetone (volume not specified). Moderate focal
hyperplasia was found in the treated group, but no tumors
developed.
The inhibitory effect of fumaric acid on hepatocarcinogenesis
was examined in male IBR mice fed 0.035% thioacetamide
in the diet for 40 weeks and then fed a basal diet for
48 weeks. The inhibitory effect of 1% fumaric acid in the
basal diet on thioacetamide carcinogenesis was so marked
that no hepatic carcinomas were found in any of the 15 animals
fed fumaric acid in combination with thioacetamide
. Similar inhibitory effects of fumaric acid on
forestomach and lung carcinogenesis in mice (that resulted
from exposure to potassium naphthyridine-3-carboxylate)
have been identified.
storage
Fumaric acid is stable although it is subject to degradation by both
aerobic and anaerobic microorganisms. When heated in sealed
vessels with water at 150–170°C it forms DL-malic acid.
The bulk material should be stored in a well-closed container in a
cool, dry place.
Purification Methods
Crystallise it from hot M HCl or water and dry it at 100o. [Beilstein 2 IV 2202.]
Incompatibilities
Fumaric acid undergoes reactions typical of an organic acid.
Incompatibilities
Dust cloud from powder or granular form mixed with air can explode. Incompatible with oxidi zers (chlorates, nitrates, peroxides, permanganates, perchlo rates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, sulfuric acid, caustics, ammonia, amines, isocyanates, alkylene oxi des; epichlorohydrin. Decomposes above 350℃ forming toxic fumes of maleic anhydride.
Waste Disposal
Use a licensed professional waste disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regula tions must be observed.
Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (oral capsules, suspensions, syrups, extended release and sustained action chewable tablets). Included in the Canadian List of Acceptable Nonmedicinal Ingredients.
Fumaric acid Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| DONBOO AMINO ACID COMPANY | +8613063595538 | donboo@donboo.com | China | 9365 | 58 |
| ZHEJIANG JIUZHOU CHEM CO., LTD | +86-0576225566889 +86-13454675544 | admin@jiuzhou-chem.com;jamie@jiuzhou-chem.com;alice@jiuzhou-chem.com | China | 12272 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81138252 +86-18789408387 | 1057@dideu.com | China | 3957 | 58 |
| Nanjing Deda New Material Technology Co., Ltd | +8613223293093 | bella@njdeda.com | China | 80 | 58 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 | sales@myuchem.com | China | 7193 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5882 | 58 |
| Hebei Fengjia New Material Co., Ltd | +86-0311-87836622 +86-17333973358 | sales06@hbduling.cn | China | 8056 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 2990 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | qinhe02@xaltbio.com | China | 1000 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 994 | 58 |
Related articles
- Fumaric acid: Applications and Production Methods
- Fumaric acid, pivotal in diverse industries, utilizes petrochemical methods for efficiency and sustainability, with emerging g....
- Jul 11,2024
- Fumaric Acid is a Naturally Occurring Dicarboxylic Acid
- As a naturally occurring dicarboxylic acid, the utilization of fumaric acids is expected to increase.
- Nov 4,2022
View Lastest Price from Fumaric acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-20 | Fumaric acid
110-17-8
|
US $900.00-700.00 / ton | 1ton | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-09-20 | Fumaric acid
110-17-8
|
US $5.00 / kg | 1kg | ≥99% | 200mt/year | Jinan Finer Chemical Co., Ltd | |
 |
2024-09-19 | Fumaric acid
110-17-8
|
US $1.60 / KG | 1KG | 98.5% | 5000kg | Hebei Chuanghai Biotechnology Co,.LTD |
-

- Fumaric acid
110-17-8
- US $900.00-700.00 / ton
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
-

- Fumaric acid
110-17-8
- US $5.00 / kg
- ≥99%
- Jinan Finer Chemical Co., Ltd
-

- Fumaric acid
110-17-8
- US $1.60 / KG
- 98.5%
- Hebei Chuanghai Biotechnology Co,.LTD