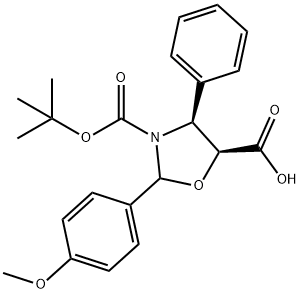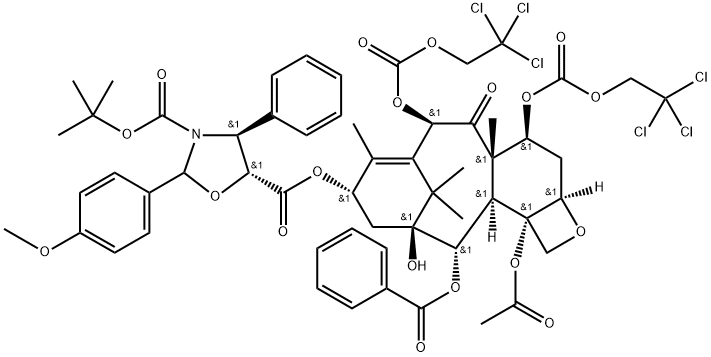
Cabazitaxel N-3 synthesis
- Product Name:Cabazitaxel N-3
- CAS Number:859498-31-0
- Molecular formula:C57H61Cl6NO19
- Molecular Weight:1276.8
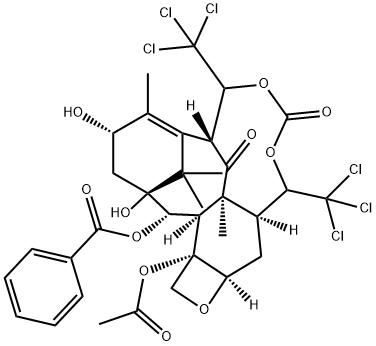
95603-44-4
50 suppliers
inquiry
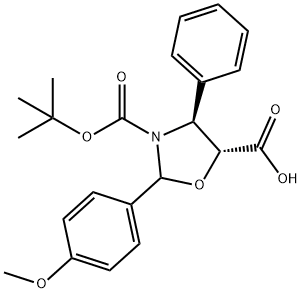
196404-55-4
187 suppliers
$8.00/250mg

859498-31-0
7 suppliers
inquiry
Yield:859498-31-0 93.9 %
Reaction Conditions:
with dmap;dicyclohexyl-carbodiimide in toluene at 10 - 20;Temperature;
Steps:
1.S2-3.S2 Step S2:
Add 78g of toluene, 0.63g of 4-dimethylaminopyridine, the above 9.1g of intermediate I and 8.8g of docetic side chain acid to the reaction kettle and stir; 6.4g N,N'-dicyclohexylcarbodiimide was dissolved with 12.75g toluene and added dropwise to the reactor, and stirred for 60 minutes after adding at a controlled temperature of 10~20 °C. After the end of the reaction, add 30.2g of purified water and 27.1g of ethyl acetate to stop the reaction, stir for about 1.5 hours, centrifuge to collect the filtrate, wash with about 30.6g of 0.2mol/L dilute hydrochloric acid, stratify after standing, extract the aqueous phase with 5.4g ethyl acetate, stratify after standing, combine the organic phase, wash twice with 9% sodium chloride solution (16.5g sodium chloride solution each time), dry the organic phase with 24g anhydrous sodium sulfate, filter, and concentrate the filtrate to oil, After adding methanol and beating, 12.2g of intermediate II was obtained by filtration and drying, and the yield was 93.90%.
References:
CN115232092,2022,A Location in patent:Paragraph 0041; 0044; 0056; 0059; 0063; 0066
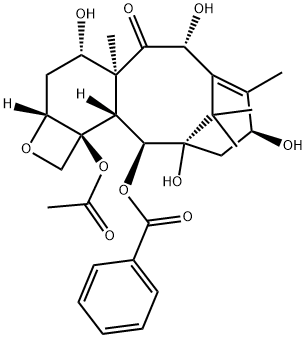
32981-86-5
536 suppliers
$9.00/25mg

859498-31-0
7 suppliers
inquiry
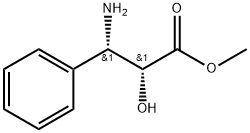
157240-36-3
16 suppliers
inquiry

859498-31-0
7 suppliers
inquiry
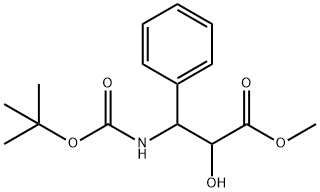
124605-42-1
168 suppliers
$8.00/250mg

859498-31-0
7 suppliers
inquiry