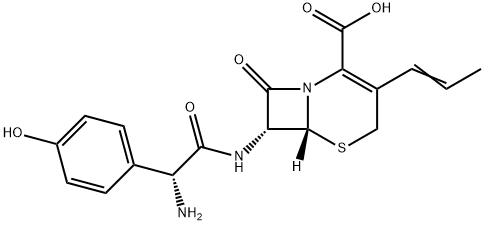
Cefprozil synthesis
- Product Name:Cefprozil
- CAS Number:92665-29-7
- Molecular formula:C18H19N3O5S
- Molecular Weight:389.43
![(6R,7R)-7-Amino-8-oxo-3-(1-propenyl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid](/CAS/GIF/120709-09-3.gif)
120709-09-3
166 suppliers
$40.00/1g
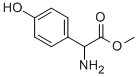
37763-23-8
205 suppliers
$10.00/1g

92665-29-7
266 suppliers
$28.00/5mg
Yield:92665-29-7 97.74%
Reaction Conditions:
Stage #1: (7R,8R)-7-amino-3-[propen-1-yl]-3-cephem-4-carboxylic acidwith penicillin acylase at 30 - 35; pH=7; for 0.5 h;Enzymatic reaction;
Stage #2: D-2-p-hydroxyphenylglycine methyl esterwith sodium lauryl sulfate in 1,4-dioxane at 30 - 35; for 2 h;Reagent/catalyst;
Steps:
4-7; 3-5 Example 4:CefprozilSynthesis
Dissolve 1.2g of immobilized penicillin acylase for synthesis in 30mL HEPES buffer(pH=7.0), after dissolution, the buffer was added to 30 mL.Add 20 mmol (4.80 g) of Compound 2 to a 250 mL Erlenmeyer flask.10mL HEPES buffer (pH = 7.0), preheated to 30 ~ 35 ° C,Mix with the enzyme filtrate and shake at 30-35 ° C for 30 min.Then add dissolved 26mmol (4.71g)L-p-hydroxyphenylglycine methyl ester1,4-dioxane 60 mL, 1 mmol sodium lauryl sulfate,It was placed in a shaking box at 30-35 ° C for 2 h, and the rotation speed was 200 rpm.The reaction pH was maintained to 6.5-7.5 with a 3 mol/L hydrochloric acid solution and a 3 mol/L sodium hydroxide solution. After completion of the reaction, the reaction mixture was separated by a 100-mesh sieve to obtain a synthetic penicillin acylase and a cefprozil reaction solution. The reaction solution was added with 3 mol/L hydrochloric acid to adjust the pH to 2.0-2.5, extracted with dichloromethane, and the aqueous phase was taken. The pH was adjusted to 5.0-5.5 by adding 3 mol/L sodium hydroxide solution, crystallizing for 30 min, suction filtration, washing,The pure cefprozil was 7.62 g, the yield was 97.74%, and the purity was 99.95%.
References:
CN109517000,2019,A Location in patent:Paragraph 0024-0031; 0036-0041
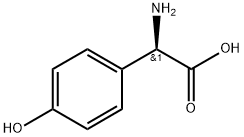
22818-40-2
444 suppliers
$7.00/10g

92665-29-7
266 suppliers
$28.00/5mg
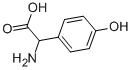
6324-01-2
4 suppliers
inquiry

92665-29-7
266 suppliers
$28.00/5mg