Cefprozil
- CAS No.
- 92665-29-7
- Chemical Name:
- Cefprozil
- Synonyms
- Procef;Prozef;Cefrozil;bmy28100;Cefprozi;CEFPROZIL;Cefprozile;Cephprozyl;Cefprozil-d4;cis-Cefprozil
- CBNumber:
- CB4739649
- Molecular Formula:
- C18H19N3O5S
- Molecular Weight:
- 389.43
- MDL Number:
- MFCD00865082
- MOL File:
- 92665-29-7.mol
- MSDS File:
- SDS
| Melting point | 215-217°C (dec.) |
|---|---|
| Boiling point | 803℃ |
| Density | 1.53±0.1 g/cm3(Predicted) |
| RTECS | XI0339000 |
| Flash point | >110°(230°F) |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | DMF: 0.3 mg/ml; DMSO: 2.5 mg/ml; PBS (pH 7.2): 1.25 mg/ml |
| form | Powder |
| pka | 2.92±0.50(Predicted) |
| Water Solubility | Soluble in DMSO at 10mg/ml. Insoluble in water. |
| BCS Class | 3 |
| InChIKey | WDLWHQDACQUCJR-PBFPGSCMSA-N |
| SMILES | N12[C@@]([H])([C@H](NC([C@H](N)C3=CC=C(O)C=C3)=O)C1=O)SCC(C=CC)=C2C(O)=O |
| CAS DataBase Reference | 92665-29-7(CAS DataBase Reference) |
| FDA UNII | 1M698F4H4E |
| ATC code | J01DC10 |
Cefprozil price More Price(24)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Alfa Aesar | J66326 | Cefprozil | 92665-29-7 | 100mg | $143 | 2024-03-01 | Buy |
| Alfa Aesar | J66326 | Cefprozil | 92665-29-7 | 1g | $407 | 2023-06-20 | Buy |
| Cayman Chemical | 23840 | Cefprozil ≥95% (mixture of cis and trans) | 92665-29-7 | 5mg | $32 | 2024-03-01 | Buy |
| Cayman Chemical | 23840 | Cefprozil ≥95% (mixture of cis and trans) | 92665-29-7 | 10mg | $57 | 2024-03-01 | Buy |
| Cayman Chemical | 23840 | Cefprozil ≥95% (mixture of cis and trans) | 92665-29-7 | 25mg | $116 | 2024-03-01 | Buy |
Cefprozil Chemical Properties,Uses,Production
Description
Cefprozil is a new orally active cephalosporin useful in the treatment of otitis media, uncomplicated skin and skin structure infections plus upper/lower respiratory tract infections. Although structurally similar to cefadroxil, its spectrum of antibacterial activity is comparable to cefaclor, but exhibits greater activity against S.aureus, S. epidermidis, Listeria monocytogenes, Streptococci, H. influenzae, Propionibacterium acnes. Clostridium perfringens and difficile.
Chemical Properties
Pale Yellow Solid
Originator
Bristol-Myers Squibb (U.S.A.)
Uses
Semisynthetic oral cephalosporin consisting of approx. 90:10 Z/E isomeric mixture. Antibacterial
Definition
ChEBI: A semisynthetic, second-generation cephalosporin, with prop-1-enyl and (R)-2-amino-2-(4-hydroxyphenyl)acetamido groups at positions 3 and 7, respectively, of the cephem skeleton. It is used to treat bronchitis as well as ear, skin and other bacterial infec ions.
Manufacturing Process
A mixture of 4-hydroxy-D-phenylglycine (10 g), ethylene glycol (15 ml) and
concentrated sulfuric acid (5 ml) was stirred for 18 hours at 55°C under
anhydrous conditions. The solution was cooled, and then ice (10 g) was added
to it, and the pH was adjusted to 1.0 with 10 N NH4OH (4.5 ml) giving 40 ml
of solution of hydroxyethyl ester of 4-hydroxy-D-phenylglycine.
The enzyme mixture of 20 ml containing immobilized recombinant penicillin G
amidase as the enzyme, 10% hydroxyethyl ester of 4-hydroxy-D-phenylglycine, 4% cefprozil (amine source), and 8% enzyme (immobilized
recombinant penicillin G amidase, equivalent to 32 IU/ml of enzyme) was
made up without buffer. The above prepared ester solution (6.9 ml) was
mixed with water (2 ml) and adjusted to pH 7.5 with 10 N NH4OH. Then the
amine source (0.8 g) was added to it and the pH adjusted to 7.5 with 1 N
NH4OH and the volume to 18.4 ml. Then the mixture was cooled to 5-15°C
and solid enzyme (1.6 g; 640 IU) was added to it. The pH was not maintained
at 7.5 and fell about 0.6 units during the reaction. The reaction mixture was
analyzed by HPLC on a C18 Reverse Phase column. The mobile phase was
10% acetonitrile/0.3% H3PO4. The isomers of cefprozil appeared at 2.9
minutes (cis) and at 5.1 minutes (trans). The final product was obtained with
a maximum yield of 92-96%. The whole experiment was completed in 25-50
min.
Synthesis of cefprozil may be carried out at 15°C using Boehringer penicillin G
amidase as the enzyme and hydroxyethyl ester of 4-hydroxy-D-phenylglycine.
A maximum yield of about 95%. The experiment was completed in 35
minutes.
brand name
Cefzil (Bristol-Myers Squibb).
Therapeutic Function
Antibiotic
Antimicrobial activity
Activity against Gram-positive cocci and Gram-negative bacilli is better than that of cefadroxil (which it structurally resembles) but is not as good as that of group 5 agents . It is moderately stable to hydrolysis by the common plasmid-mediated β-lactamases, but is hydrolyzed by the chromosomal enzymes of Gram-negative bacilli .
Hazard
Moderately toxic by ingestion. Human sys- temic effects.
Pharmacokinetics
Oral absorption: >90%
Cmax 250 mg oral: 5–7 mg/L after 1 h
500 mg oral: 10 mg/L after 1
Plasma half-life: 1–1.4 h
Volume of distribution: 15–201
Plasma protein binding: 35–45%
Absorption and distribution
It is almost completely absorbed and well distributed, penetrating
well into tonsillar and other tissues and inflammatory
exudate. Absorption is unaffected by food or
antacids and there is no accumulation on multiple dosing
regimens.
Metabolism and excretion
Most of the dose is excreted unchanged in urine, though
about 20% is found in feces. Urinary concentrations after a
500 mg oral dose usually exceed 1 g/L. The elimination halflife
is prolonged in patients with renal impairment, reaching
6 h in anuric patients. About half the drug is removed in 3 h
by hemodialysis.
Pharmacokinetics
Oral absorption: >90%
Cmax 250 mg oral: 5–7 mg/L after 1 h
500 mg oral: 10 mg/L after 1
Plasma half-life: 1–1.4 h
Volume of distribution: 15–201
Plasma protein binding: 35–45%
Absorption and distribution
It is almost completely absorbed and well distributed, penetrating
well into tonsillar and other tissues and inflammatory
exudate. Absorption is unaffected by food or
antacids and there is no accumulation on multiple dosing
regimens.
Metabolism and excretion
Most of the dose is excreted unchanged in urine, though
about 20% is found in feces. Urinary concentrations after a
500 mg oral dose usually exceed 1 g/L. The elimination halflife
is prolonged in patients with renal impairment, reaching
6 h in anuric patients. About half the drug is removed in 3 h
by hemodialysis.
Clinical Use
Cefprozil (Cefzil) is an orally active second-generationcephalosporin that is similar in structure and antibacterialspectrum to cefadroxil. Oral absorption is excellent (oralbioavailability is about 95%) and is not affected by antacidsor histamine H2-antagonists. Cefprozil exhibits greater invitro activity against streptococci, Neisseria spp., and S. aureusthan does cefadroxil. It is also more active than thefirst-generation cephalosporins against members of theEnterobacteriaceae family, such as E. coli, Klebsiella spp., P. mirabilis, and Citrobacter spp. The plasma half-life of1.2 to 1.4 hours permits twice-a-day dosing for the treatmentof most community-acquired respiratory and urinary tractinfections caused by susceptible organisms.
Clinical Use
It has been used for various infections for which oral cephalosporins are appropriate.
Clinical Use
As for group 2 cephalosporins . It should not be used in infections in which H. influenzae is, or is likely to be, implicated. It should not be used as an alternative to penicillin in syphilis.
Side effects
Nausea, vomiting and abdominal discomfort are relatively common. Pseudomembranous colitis has been described and overgrowth of Candida with vaginitis may be troublesome. Otherwise, mild hypersensitivity reactions and biochemical changes common to cephalosporins occur. Very rare neurological disturbances have been described, particularly in patients in whom very high plasma levels have been achieved. There are rare reports of Stevens–Johnson syndrome and toxic epidermal necrolysis.
Side effects
It is well tolerated. Diarrhea and gastrointestinal discomfort may occur. There have been a few reports of pseudomembranous colitis and serum sickness-like reactions.
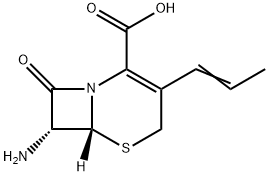
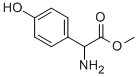
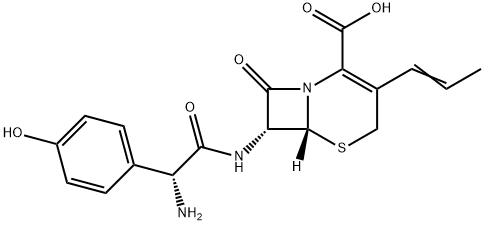
Cefprozil Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Lewoo Pharmatech(Shanghai)Co.,Ltd | +86-15800805830 +86-15800805830 | miao@lewoopharma.com.cn | China | 156 | 58 |
| Shenzhen Excellent Biomedical Technology Co.,Ltd. | +86-0755-26050679 +86-15915472436 | sale@ex-biotech.cn | China | 1031 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 | sarah@tnjone.com | China | 900 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| Hebei Runbin Biotechnology Co. LTD | 13180553332 | 2179877681@qq.com | CHINA | 996 | 58 |
Related articles
- Pharmacokinetic and bioequivalence study of cefprozil suspension granules
- Cefprozil is an oral second-generation semisynthetic cephalosporin with broad-spectrum antibacterial activity.
- Dec 1,2023
- Cefprozil: Antimicrobial Activity, Susceptibility, Administration and Dosage, Clinical Uses etc.
- Cefprozil has sometimes been described as a third-generation cephalosporin but its spectrum of activity is only slightly wider....
- Mar 22,2022
View Lastest Price from Cefprozil manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-10 | Cefprozil
92665-29-7
|
US $0.00-0.00 / mg | 10mg | 90%+ | 10g | Guangzhou PI PI BIOTECH INC | |
 |
2024-04-09 | Cefprozil
92665-29-7
|
US $0.00 / Kg | 1Kg | 99% | 10kg | Shaanxi TNJONE Pharmaceutical Co., Ltd | |
 |
2023-09-05 | Cefprozil
92665-29-7
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd |





