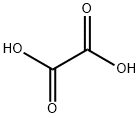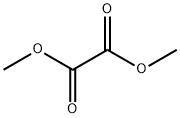
Dimethyl oxalate synthesis
- Product Name:Dimethyl oxalate
- CAS Number:553-90-2
- Molecular formula:C4H6O4
- Molecular Weight:118.09
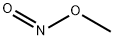
624-91-9
11 suppliers
inquiry
201230-82-2
1 suppliers
inquiry

553-90-2
314 suppliers
$10.00/5g
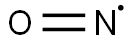
10102-43-9
56 suppliers
$479.00/1Kit
Yield:553-90-2 98.9%
Reaction Conditions:
with 0.5% Pd/C at 100; under 375.038 Torr; for 0.000555556 h;Pressure;Reagent/catalyst;Temperature;Time;
Steps:
1 Example 1
Example 1[0032]Sodium nitrite, water and inorganic acid first enter a reactor I to produce an NO containing effluent I; an effluent II of NO is then obtained by separating the effluent I; the effluent II of NO and methanol and oxygen enter a reactor. II to be subjected to the oxidative esterification reaction to produce an effluent III comprising methyl nitrite; said effluent III is separated to obtain the effluent IV of methyl nitrite; said effluent IV of methyl nitrite and a CO gas stream enter a coupling reactor where they are contacted with a Pd-containing catalyst and reacted to produce an effluent V of oxalate and an NO containing effluent VI; the NO containing effluent VI is returned to an entrance of the reactor II for being mixed with the effluent II of NO and then continuing to participate into the reaction; here, both the reactor I and the reactor II are rotating supergravity reactors; Pd-containing catalyst contains Pd in an amount of 0.5 wt. % based on the weight of the catalyst support. For the reactor I, the molar ratio of water to sodium nitrite is 2:1; the molar ratio of sulphuric acid to sodium nitrite is 0.5:1; the reaction temperature is 10 C; the reaction pressure is -0.05 MPa; the reaction contacting time is 0.05 s. For the reactor II, the reaction temperature is 30° C.; the reaction pressure is -0.05 MPa; the reaction contacting time is 0.02 s; the molar ratio of NO in the NO effluent II and the NO containing effluent VI, methanol and oxygen is 1:2:0.2; the coupling reactor has a reaction temperature 100° C.; a reaction contacting time is 2 s; a reaction pressure is -0.05 MPa; a molar ratio of CO in the CO gas stream to methyl nitrite is 1.5:1; the rotor of the rotating supergravity reactor of the reactor I and the reactor II has a rotational speed of 600 rpm. The results are: a selectivity of NO of the reactor I of 99.99%, the selectivity of the nitrous acid ester of the reactor II of 98.9%, a space time yield of dimethyl oxalate of the coupling reactor of 860 g/(h.L); and a selectivity of dimethyl oxalate of 98.9%.
References:
US2013/79549,2013,A1 Location in patent:Paragraph 0032

67-56-1
727 suppliers
$9.00/25ml

79-37-8
461 suppliers
$17.67/10gm:

553-90-2
314 suppliers
$10.00/5g

67-56-1
727 suppliers
$9.00/25ml

124-38-9
121 suppliers
$214.00/14L
201230-82-2
1 suppliers
inquiry

553-90-2
314 suppliers
$10.00/5g

67-56-1
727 suppliers
$9.00/25ml

124-38-9
121 suppliers
$214.00/14L
201230-82-2
1 suppliers
inquiry
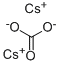
534-17-8
590 suppliers
$5.00/5g

553-90-2
314 suppliers
$10.00/5g
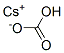
15519-28-5
13 suppliers
inquiry
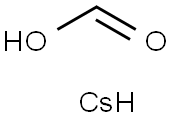
3495-36-1
135 suppliers
$11.00/5g