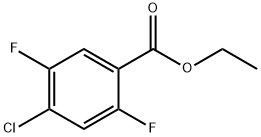
Ethyl 4-chloro-2,5-difluorobenzoate synthesis
- Product Name:Ethyl 4-chloro-2,5-difluorobenzoate
- CAS Number:879093-03-5
- Molecular formula:C9H7ClF2O2
- Molecular Weight:220.6
132794-07-1
170 suppliers
$5.00/1g

64-17-5
699 suppliers
$10.00/50g

879093-03-5
15 suppliers
$91.00/500mg
Yield:879093-03-5 94%
Reaction Conditions:
sulfuric acid for 18 h;Heating / reflux;
Steps:
2; IIIB
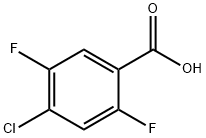
Sulfuric acid (62.4 ml, 1.13 moles) was added to a solution of 2,5-difluoro-4-chloro-benzoic acid (750.0 g, 3.895 moles) (PLEASE DEFINE THE SOURCE OF THIS REACTANT) in absolute ethanol (3.75 L). The mixture was refluxed for 18 hours. The mixture was concentrated to remove most of ethanol and then cooled to room temperature. The residue was neutralized with 1N aqueous NaOH (2.4 L) and extracted with ethyl acetate (2×1L). The combined extracts were washed with sat. aqueous NaHCO3 and brine, then dried with (MgSO4) and concentrated to dryness. Ethyl 2,5-difluoro-4-chloro-benzoate (806.7 g) was obtained at 94% yield. 1H NMR (in CDCl3): δ7.72 (dd, J=9.1/6.2, 1H), 7.22 (dd, J=9.1/5.6, 1H), 4.39 (q, 3J=7.0, 2H), 1.39 (t, J=7.0, 3H). GC-MS: 161 (M+).
References:
US2007/21634,2007,A1 Location in patent:Page/Page column 4; 6