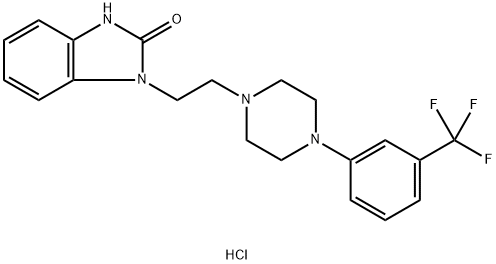
Flibanserin Hydrochloride synthesis
- Product Name:Flibanserin Hydrochloride
- CAS Number:147359-76-0
- Molecular formula:C20H22ClF3N4O
- Molecular Weight:426.87
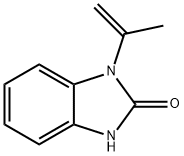
52099-72-6
204 suppliers
$5.00/250mg
![1-(2-CHLOROETHYL)-4-[3-(TRIFLUOROMETHYL)PHENYL]PIPERAZINE DIHYDROCHLORIDE](/CAS/GIF/57061-71-9.gif)
57061-71-9
26 suppliers
inquiry

147359-76-0
93 suppliers
inquiry
Yield:147359-76-0 77%
Reaction Conditions:
Stage #1: 1,3-dihydro-1-(1-methylethenyl)-2H-benzimidazol-2-one;1-chloro-2-[4-(3-trifluoromethylphenyl)piperazin-1-yl]ethanewith potassium carbonate in dimethyl sulfoxide at 58; for 8 h;Large scale;
Stage #2: with hydrogenchloride in ethanol;water at 65; for 2 h;Large scale;Temperature;
Steps:
1.4; 2.4; 3.4 4. Synthesis of the compound flibanserin hydrochloride
10-1-G 2.285KG, 10-1-B 4.434KG, K2CO35.17KG and 18L of dimethyl sulfoxide were added to a 50L oil bath, and reacted at 58°C for 8 hours. TLC showed that the reaction of the raw materials was complete. Pour into water, extract 3 times with dichloromethane, combine the organic phases, wash 3 times with water, wash once with saturated brine, dry with anhydrous sodium sulfate, and spin dry to obtain a gray solid.The gray solid was dissolved by heating with 25L ethanol, 6L concentrated HCl was added and reacted at 65°C for 2 hours. After the reaction was completed, the reaction solution was released. 15L ethanol was added and cooled to room temperature. The solid was precipitated, filtered with suction, and rinsed with ethanol to obtain 4.4kg of white solid. The product was confirmed by NMR, and the yield was 77%.
References:
CN111303043,2020,A Location in patent:Page/Page column 6-8

52099-72-6
204 suppliers
$5.00/250mg

147359-76-0
93 suppliers
inquiry
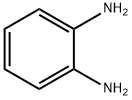
95-54-5
486 suppliers
$9.00/1g

147359-76-0
93 suppliers
inquiry
![1-(2-bromoethyl)-3-(prop-1-en-2-yl)-1H-benzo[d]imidazol-2(3H)-one](/CAS/20180703/GIF/136081-17-9.gif)
136081-17-9
11 suppliers
inquiry

147359-76-0
93 suppliers
inquiry
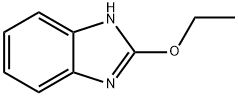
22219-23-4
29 suppliers
$183.00/250mg

147359-76-0
93 suppliers
inquiry