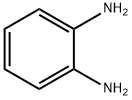
o-Phenylenediamine
- CAS No.
- 95-54-5
- Chemical Name:
- o-Phenylenediamine
- Synonyms
- benzene-1,2-diamine;OPDA;Phenylenediamine;OPD;1,2-PHENYLENEDIAMINE;1,2-DIAMINOBENZENE;orthamine;1,2-BENZENEDIAMINE;ORTHO-TOLUENEDIAMINE;ORTHOPHENYLENEDIAMINE
- CBNumber:
- CB9854699
- Molecular Formula:
- C6H8N2
- Molecular Weight:
- 108.14
- MDL Number:
- MFCD00007721
- MOL File:
- 95-54-5.mol
- MSDS File:
- SDS
| Melting point | 100-102 °C |
|---|---|
| Boiling point | 256-258 °C |
| Density | 1,27 g/cm3 |
| vapor density | 3.7 (vs air) |
| vapor pressure | 0.01 mm Hg ( 25 °C) |
| refractive index | 1.6339 (estimate) |
| Flash point | 110°C |
| storage temp. | 2-8°C |
| solubility | H2O: 1 tablet/10 mL, clear, colorless |
| form | tablet |
| Colour Index | 76010 |
| pka | 4.46(at 25℃) |
| color | white to off-white |
| PH Range | Green Q uorescence (3.1) to nonQ uorescence (4.4) |
| PH | 7-8 (50g/l, H2O, 20℃) |
| explosive limit | 1.5%(V) |
| Water Solubility | <0.1 g/100 mL at 20 ºC |
| Sensitive | Air & Light Sensitive |
| Merck | 14,7284 |
| BRN | 606074 |
| Exposure limits | TLV-TWA 0.1 mg/m3; carcinogenicity: A2- Suspected Human Carcinogen (ACGIH 1989). |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| Major Application | bottom antireflective coatings, semiconductor devices, hair dyes, detecting antibody, substrate for horseantibody, glucose, streptomycin, penicillin, sulfonamide, neuroglobin, substrate for horseradish peroxidase |
| LogP | 0.12-0.17 at 20-25℃ and pH7-8 |
| CAS DataBase Reference | 95-54-5(CAS DataBase Reference) |
| EWG's Food Scores | 5-8 |
| FDA UNII | 8B713N8Q0F |
| NIST Chemistry Reference | 1,2-Benzenediamine(95-54-5) |
| IARC | 2B (Vol. 123) 2020 |
| EPA Substance Registry System | 1,2-Phenylenediamine (95-54-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H317-H319-H341-H351-H410 | |||||||||
| Precautionary statements | P202-P273-P280-P301+P310-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | T,N | |||||||||
| Risk Statements | 20/21-25-36-40-43-50/53-68 | |||||||||
| Safety Statements | 28-36/37-45-60-61-1/2 | |||||||||
| RIDADR | UN 1673 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | SS7875000 | |||||||||
| F | 8-10-23 | |||||||||
| Autoignition Temperature | 540 °C | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29215119 | |||||||||
| Toxicity | LD50 in rats (mg/kg): 1070 orally; 516 i.p., C. Burnett et al., J. Toxicol. Environ. Health 2, 657 (1977) | |||||||||
| NFPA 704 |
|
o-Phenylenediamine price More Price(45)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | P5412 | o-Phenylenediamine tablet, 20 mg substrate per tablet | 95-54-5 | 50tablets | $261 | 2024-03-01 | Buy |
| Sigma-Aldrich | P5412 | o-Phenylenediamine tablet, 20 mg substrate per tablet | 95-54-5 | 100tablets | $481 | 2024-03-01 | Buy |
| Sigma-Aldrich | P23938 | o-Phenylenediamine flaked, 99.5% | 95-54-5 | 5g | $36.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.14538 | 1,2-Phenylenediamine for synthesis | 95-54-5 | 5G | $32.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.14538 | 1,2-Phenylenediamine for synthesis | 95-54-5 | 250g | $55.5 | 2024-03-01 | Buy |
o-Phenylenediamine Chemical Properties,Uses,Production
Phenylenediamine
Phenylenediamine is the simplest aromatic diamine. There are three isomers, namely o-phenylenediamine, m-phenylenediamine and p-phenylenediamine.
p-Phenylenediamine appears as a colorless crystal, being quickly oxidized in the air into black. It has a boiling point of 267 °C. It is soluble in water, ethanol and ether. It can be used as raw materials or intermediates of dyes, vulcanization accelerators for rubber, developers for the photographic industry, fur dyes and hair dyes. People in frequent contact include usually dyeing hair workers, hairdressers and regular hair coloring patients. It can enter the body through the skin, being oxidized intermediates with different degrees of sensitization, being able to cause eczema-like or moss-like dermatitis, appearing mostly in the face, neck and forearm and other skin folds. For example, mouth, eye and nose and other parts are easy to be damaged. People inhalation of its dust can obtain bronchial asthma. The symptoms of oral poisoning are similar as aniline poisoning, being mainly subject to symptomatic treatment.
O-phenylenediamine is also known as 1, 2-diaminobenzene, 1, 2-phenylenediamine. Substance precipitated from the water appears as white to pale yellow leaf-like crystals while product precipitated from chloroform appears as prism-like crystals. Its color is easy to change upon exposure to air, from white to yellow, brown, purple, and finally into black. It has a relative density of 1.2698, the melting point of 103 °C to 104 °C, the boiling point of 256~258 °C. It is slightly soluble in cold water (35 °C 4.15), being easily soluble in hot water (81 °C, 733), ethanol, ether, chloroform and benzene. Its reaction with inorganic acid can lead to the formation of water-soluble salts; its aqueous solution can react with carbon disulfide to generate 2-mercapto-benzimidazole; under pressure, it can react with carbon dioxide reaction to generate benzimidazolone. This product is toxic with inhalation causing visual disturbances. Its contact with the skin can cause inflammation with entering into the eyes being able to cause inflammation as well. Rat oral LD50: 1070mg/kg.
M-phenylenediamine appears as a rhombic crystal, being quickly oxidized to brown color in the air. Its proportion is 1.139 with the melting point of 62.8 °C and the boiling point of 284 °C. It can be dissolved in water, alcohols, and ketones and so on. It can be used in the dye chemical industry, and as a curing agent for plastics. It can also enter into the human body via the respiratory tract, skin and gastrointestinal tract. Acute poisoning can cause methemoglobinemia, and liver damage and hemolytic anemia.
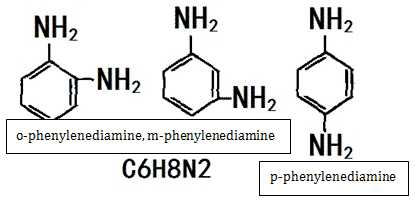
Figure 1 is the chemical structure of three isomers of phenylenediamine: o-phenylenediamine, m-phenylenediamine, p-phenylenediamine.
Chemical properties
O-phenylenediamine has active chemical properties, being able to have condensation reaction with acid, aldehydes, ketones and other compounds, generating heterocyclic compounds; it can also have oxidation, condensation, substitution and diazotization reaction.
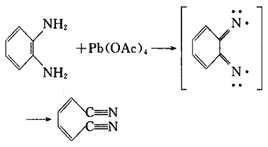
Figure 2 is o-phenylenediamine oxidation.
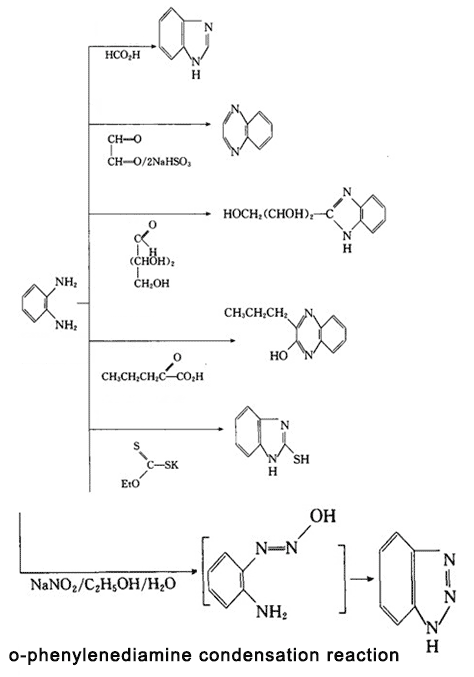
Figure 3 is o-phenylenediamine condensation reaction.
The main purpose and role of phenylenediamine
Phenylenediamine is used as a fluorescent indicator and chromatographic analysis reagent. It is used for the identification of copper, gold, iron, magnesium, vanadium, ammonia, hydrogen sulfide, sulfur dioxide and chromic oxide, etc., and manufacturing azo dyes, fur dyeing and photographic materials. , Printing and dyeing industry.
(1) o-phenylenediamine is mainly used in the manufacture of pesticides fungicides (carbendazim, benomyl, thiophanate-methyl, Thiabendazole), reducing dyes (reduction Yellow 6GD, reduction brilliant orange GR), cationic dye (Cationic brilliant yellow 10GFF), polymer stabilizer (2-mercaptobenzimidazole), heterocyclic compounds (benzimidazole and quinoxaline), photographic materials, surfactants, antifreeze, copper anticorrosive. It is one of the components of the hair dye formulation and is one of the organic reagents commonly used in analytical chemistry to identify 1, 2-diesters, carboxylic acids and aldehydes.
(2) m-phenylenediamine is mainly used in the manufacture of dyes, such as direct fast black RN, and used as fur dyes, azo and diazine dye intermediates, the determination of nitrite, but also for ion exchange resins, block copolymer and photographic; for textile dyes, laboratory reagents, curing agents, corrosion inhibitors. Also used as a curing agent for epoxy resin and cement accelerator and so on.
(3) p-phenylenediamine is mainly used in the manufacture of dyes, being also used for the synthesis of azo dyes disperse dyes, acid dyes, direct dyes, sulfur dyes, fur dyes (fur D). It can also be used for cosmetics Hair dye, also used in the preparation of rubber antioxidant ("anti-aging agent DNP", "anti-aging agent DOP", "anti-aging agent DBP") and the production of developing agents.
Toxicity
O-phenylenediamine has moderate toxicity, often due to inhalation of dust or skin absorption caused by poisoning (the latter phenomenon mostly). Inhalation of dust can cause rhinitis, bronchitis, frequent fever, causing unique asthma and vagal nervous tension caused by the tracheal inflammation, can cause severe acute eczema and eczema. M-phenylenediamine can cause methemoglobin changes, while the p-phenylenediamine can cause bronchial asthma, allergic dermatitis.
The above information is edited and edited by Chemicalbook.
Uses
1. It can be used as a cationic dye intermediates, being the main raw material of pesticides, carbendazim and other fungicides.
2. It can be used as analytical reagent, fluorescent indicator, also used in organic and dye synthesis. It can be used as pesticides, pharmaceuticals and dyes intermediates
3. As a pesticide intermediates, dye intermediates
phenylenediamine is the intermediate of fungicide carbendazim, methyl thiophanate, and thiabendazole, but also insecticides quinalphos intermediates. In addition, it can also used as an important intermediate of dye industry.
4. The goods are intermediates of dyes, pesticides, additives and photographic materials. Itself is the dye fur yellow brown M. It can be used in the manufacture of polyamide, polyurethane, fungicides carbendazim and thiophanate, reduction scarlet GG, leveling agent, antioxidant MB, also used in the preparation of the developer, surfactant and so on.
5. It can be used as intermediates of chemical raw materials, pesticides and dyes.
Production method
Take o-nitroaniline as raw materials, use sodium sulfide reduction or catalytic hydrogenation for reduction of o-phenylenediamine. Addition of 21% sodium sulfide solution and o-nitroaniline into the reducing pot, raising the temperature to 90 °C, close and raise the temperature to 105-110 °C and increase the pressure to 0.1-0.2 MPa, stirring and reducing for 5 h, cooling to below 40 °C, filter. The filter cake is heated and melted, vacuum distillation, collecting the fraction in 140-210 °C (7.89kPa), namely o-phenylenediamine with the yield of 70-80%. The hydrogenation process should proceed in the autoclave with the reaction temperature of 95-105 °C with the pressure of about 2MPa. It should be subject to hydrogenation reaction under the action of nickel catalyst for about 1h until the hydrogen pressure does not decline. The yield of o-phenylenediamine was above 97%.
There are several preparation methods as follows.
O-Nitroaniline and Sodium Sulfide Reduction
Its reaction with o-nitroaniline and sodium sulfide solution at a pressure of 98 to 196 kPa and a reaction temperature of 105 to 110 ° C, being also carried out at a reaction temperature of 120 to 130 °C under atmospheric pressure for 3 to 4 hours to obtain o-benzenediamine amine.
O-nitroaniline: hydrogenation reduction method
Use o-nitroaniline as the raw material and Raney nickel as the catalyst, the reaction temperature was raised from 95 to 105 ° C in the autoclave, and the product was obtained by stirring at 1.960 MPa under continuous pressure of hydrogen gas until the pressure did not drop.
For the above two preparation methods, the sodium sulfide reduction method can obtain the product of high purity with relative mature process. There are more factories in the domestic production of o-phenylenediamine. But the method has poor labor protection conditions with much wastewater. There are still some problems in the catalytic hydrogenation with industrialization needs to be further improved. In addition, there is also o-dichlorobenzene ammonia solution, there are more units have been studied, but not yet industrialized.
Chemical properties
It appears as colorless monoclinic crystal with air and sunlight in the darker. It is slightly soluble in cold water, being more soluble in hot water, soluble in alcohol, ether and chloroform.
Hazards & Safety Information
Category Toxic substances
Toxicity classification highly toxic
Acute toxicity Oral-Rat LD50: 1070 mg/kg; Oral-mouse LD50: 366 mg/kg
Flammability hazardous characteristics it is flammable with heating releasing toxic aniline gases
Storage and transportation characteristics Treasury: ventilated, low-temperature and dry; store it separately from food raw materials for storage and transportation
Fire extinguishing agent mist water, carbon dioxide, sand
Chemical Properties
White to off white solid
Chemical Properties
o-Phenylenediamine is a white to brownish crystalline substance that turns red upon exposure to air.
Uses
o-Phenylenediamine is an amino substituted benzene used in the manufacture of dyes. Potential use in sensitive immunosensor for cancer biomarker.Environmental toxin on US EPA Toxic Release Inventory list (TRI) list.
Uses
m-Phenylenediamine is used in the manu facture of a variety of dyes, in hair dyeformulations, as a rubber curing agent, inpetroleum additives, as a photographic developing agent, and as an analytical reagent.
Uses
o-Phenylenediamine is used as an intermediate in the manufacture of dyes, pigments, andfungicides. It is also used in hair dye formulation, as a photographic developing agent,and in organic synthesis.
Uses
It is actively involved in the preparation of pharmaceuticals by reacting substituted o-phenylenediamine with various diketones.
Definition
ChEBI: A phenylenediamine in which the two amino groups are ortho to each other.
Synthesis Reference(s)
Organic Syntheses, Coll. Vol. 2, p. 501, 1943
Synthetic Communications, 25, p. 4025, 1995 DOI: 10.1080/00397919508011478
General Description
Colorless monoclinic crystals if pure; technical grade brownish-yellow crystals or a sandy brown solid. Used in manufacture of dyes, photography, organic synthesis.
Air & Water Reactions
Soluble in water [Hawley]. Melting point 219 F. Flash point 277 F. Toxic by skin absorption, inhalation or ingestion. Even as a solid will spot downwind area brown/purple (Roger Patrick, DuPont Engineer).
Reactivity Profile
o-Phenylenediamine a weak aromatic amine base neutralizes acids in exothermic reactions to form salts. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Darkens on exposure to air (Roger Patrick, DuPont Engineer).
Health Hazard
The toxic effects of m-phenylenediamineare similar to its ortho- and para-isomers.The toxic symptoms were tremor, excite ment, convulsions, and cyanosis, and col lapse at fatal dose. Other symptoms includeddecreased blood pressure and pulse rate.Chronic toxic effects resulting from the oralapplication of an aqueous solution of thiscompound were increase in liver and to alesser extent, kidney weights. No carcinogenicity was observed in test animals. Thecompound was excreted rapidly, unchanged.
LD50 value, oral (rats): 650 mg/kg.
Fire Hazard
Flash point data for o-Phenylenediamine are not available. o-Phenylenediamine is probably combustible.
Biochem/physiol Actions
o-Phenylenediamine (o-PD) is a potent substrate for spectrophotometric horseradish peroxidase (HRP)-mediated enzyme-linked immunosorbent assay (ELISA), which gives 2,3-diaminophenazine (DAP) as a product of o-PD oxidation.
Safety Profile
Confirmed carcinogen. Poison by ingestion and intraperitoneal routes. Moderately toxic by subcutaneous route. Mildly toxic by skin contact. Mutation data reported. A pesticide and pharmaceutical. When heated to decomposition it emits toxic fumes of NOx. See also other phenylenediamine entries and AMINES
Potential Exposure
Used as an intermediate in the making of dyes; pesticides, pharmaceuticals, and rubber chemicals; in making fungicides and other chemicals; in photographic and analytical procedures and processes
Carcinogenicity
o-PDA (dihydrochloride) induced hepatocellular carcinomas during an 18-month feeding study in male Charles River CD rats given diets containing 2000 or 4000 ppm of the chemical (female rats not used in this study). No significant carcinogenic effects were observed in male and female CD-1 mice given higher doses (6872 or 13,743 ppm) of the compound.
Shipping
UN1673 Phenylenediamines (o-, m-, p-), Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Crystallise the diamine from aqueous 1% sodium hydrosulfite (charcoal), wash it with ice-water and dry it in a vacuum desiccator, or sublime it in vacuo. It has been purified by recrystallisation from toluene and zone refined [Anson et al. J Am Chem Soc 108 6593 1986]. Purification by refluxing a CH2Cl2 solution containing charcoal is also carried out followed by evaporation and recrystallisation [Koola & Kochi J Org Chem 52 4545 1987], protect from light. The acetate has m 186o. [Beilstein 13 IV 38.]
Incompatibilities
Dust may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, acid chlorides; acid anhydrides; chloroformates. Heat and light contribute to instability. Keep away from metals; 1,2-PHENYLENEDIAMINE a weak aromatic amine base neutralizes acids in exothermic reactions to form salts. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Darkens on exposure to air (Roger Patrick, DuPont Engineer).
Waste Disposal
Controlled incineration whereby oxides of nitrogen are removed from the effluent gas by scrubber, catalytic or thermal device.
o-Phenylenediamine Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Masteam Bio-tech Co., Ltd. | +86-15200345168 +86-15200345168 | bruce.lei@masteam.com | CHINA | 153 | 58 |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | sales@molcore.com | China | 49739 | 58 |
| Guangzhou Tosun Pharmaceutical Limited | +8618922120635 | sales@toref-standards.com | China | 1000 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
Related articles
- o-Phenylenediamine: applications in fluorescent and colorimetric sensors
- o-Phenylenediamine is used to develop sensors for detecting heavy metal ions and organic molecules, offering high sensitivity ....
- Jul 12,2023
- Application and Pharmacology of o-Phenylenediamine
- 1,2-phenylenediamine appears as colorless monoclinic crystals if pure; technical grade brownish-yellow crystals or a sandy bro....
- Mar 30,2022
View Lastest Price from o-Phenylenediamine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-26 | o-Phenylenediamine
95-54-5
|
US $0.00 / Kg/Drum | 1KG | 99% | 200mt/year | Jinan Finer Chemical Co., Ltd | |
 |
2024-04-11 | Lansoprazole Impurity 22
95-54-5
|
US $0.00-0.00 / mg | 10mg | above 98% | 10g/month | Guangzhou Tosun Pharmaceutical Limited | |
 |
2024-03-31 | o-Phenylenediamine
95-54-5
|
US $40.00-30.00 / kg | 1kg | 99% | 2000000 | Shanghai Chinqesen Biotechnology Co., Ltd. |
-

- o-Phenylenediamine
95-54-5
- US $0.00 / Kg/Drum
- 99%
- Jinan Finer Chemical Co., Ltd
-

- Lansoprazole Impurity 22
95-54-5
- US $0.00-0.00 / mg
- above 98%
- Guangzhou Tosun Pharmaceutical Limited
-

- o-Phenylenediamine
95-54-5
- US $40.00-30.00 / kg
- 99%
- Shanghai Chinqesen Biotechnology Co., Ltd.