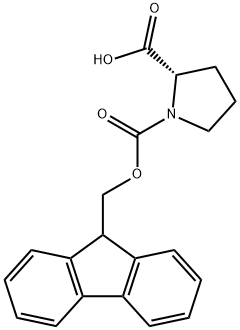
Fmoc-Pro-OH synthesis
- Product Name:Fmoc-Pro-OH
- CAS Number:71989-31-6
- Molecular formula:C20H19NO4
- Molecular Weight:337.37
28920-43-6
520 suppliers
$5.00/5g
147-85-3
967 suppliers
$6.00/25g

71989-31-6
481 suppliers
$6.00/5g
Yield:71989-31-6 95%
Reaction Conditions:
with potassium carbonate in 1,4-dioxane;water at 0 - 20;
Steps:
17.A Step A: (S)-1-((( 9H-fluoren-9-yl)methoxy)carbonyl)pyrrolidine-2-carboxylic acid
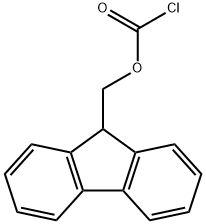
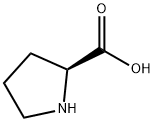
To a solution of (5')-pyrrolidine-2-carboxylic acid (1 g, 8.7 mmol) in 1,4-dioxane (4 mL) and H20 (15 mL) was added K2C03 (3.24 g, 23 mmol) followed by (9H-fluoren-9-yl)methyl chloroformate (2.3 g, 8.3 mmol) at 0 °C. The mixture was stirred at r.t. overnight and treated by H20 (10 mL). The resulting mixture was extracted with Et20 (2 x 20 mL). The aqueous phase was acidified with aq. HC1 (1 M) to pH=2-3, and extracted with DCM (3 x 50 mL). The combined organic layers were dried over anhydrous a2S04 and concentrated to give the desired product as a white solid (2.8 g, 95% yield). MS: 338 (M+l)+.
References:
AGIOS PHARMACEUTICALS, INC.;Popovici-Muller, Janeta;SAUNDERS, Jeffrey, O.;SALITURO, Francesco, G.;CAI, Zhenwei;YAN, Shunqi;ZHOU, Ding WO2013/107405, 2013, A1 Location in patent:Page/Page column 95
82911-69-1
596 suppliers
$7.00/25g

147-85-3
967 suppliers
$6.00/25g

71989-31-6
481 suppliers
$6.00/5g
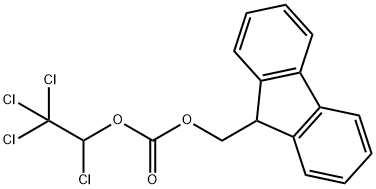
100821-63-4
0 suppliers
inquiry
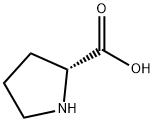
344-25-2
587 suppliers
$5.00/250mg

71989-31-6
481 suppliers
$6.00/5g
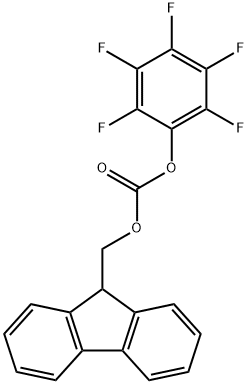
88744-04-1
125 suppliers
$24.80/1g

147-85-3
967 suppliers
$6.00/25g

71989-31-6
481 suppliers
$6.00/5g

28920-43-6
520 suppliers
$5.00/5g
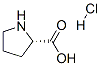
7776-34-3
38 suppliers
$63.20/5ml-f

71989-31-6
481 suppliers
$6.00/5g