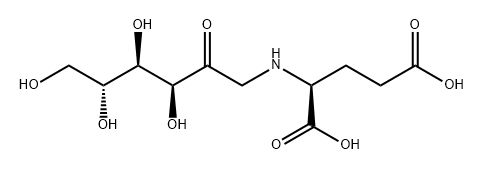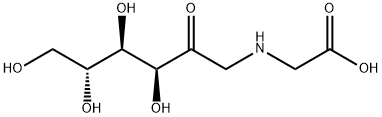
fructosyl-glycine synthesis
- Product Name:fructosyl-glycine
- CAS Number:4429-05-4
- Molecular formula:C8H15NO7
- Molecular Weight:237.21
Yield:4429-05-4 22%
Reaction Conditions:
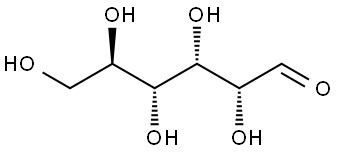
Stage #1: D-glucosewith sodium hydrogensulfite in methanol;glycerol; for 0.5 h;Reflux;
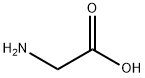
Stage #2: glycinewith acetic acid in methanol;glycerol;Reflux;
Steps:
Preparation of N-(1-deoxy-D-fructos-1-yl)-glycine
The procedure for the synthesis of fructose-glycine was as described in [1]: A suspension of 36 g (0.2 moles) of D-glucose and 2.0 g sodium bisulfite in 60 mL of methanol and 30 mL of glycerol was refluxed for 30 min, followed by the addition of 6 g (0.08 moles) of glycine and 8 mL of acetic acid. This solution was refluxed until > 80% of the amino acid had reacted, as evidenced by TLC. The resulting brown, syrupy solution was diluted with 1 volume of water, loaded on a 2 x 30 cm column of Amberlite IRN-77 (H+) ion-exchange resin, and the column was eluted with 500 mL of water, followed by 0.2 N ammonium hydroxide. Fractions of 25 mL were collected. Early fractions contained D-glucose, uncharged pigments and D-glucose-derived degradation products. The Amadori compound, along with unreacted amino acid, usually eluted near the end of the water wash and at the beginning of the ammonium hydroxide wash. The combined fractions, which contained fructose-glycine, were evaporated to 100 mL in vacuo and decolorized with charcoal (2.0 g). This solution was placed on a second 2 x 30 cm column of Amberlite IRN-77 (pyridinium form, pretreated with 10 mL of acetic acid). The column was eluted with water, and 25-mL fractions were collected. Fractions containing fructose-glycine were evaporated in vacuo at 30°C to a syrup. The syrup was diluted in methanol to turbidity and left for 3 days to crystallize at room temperature. Resulting colorless prisms were washed with 3:1 methanol-water and dried in vacuo over calcium chloride. An additional crop of crystals was obtained from the mother liquor. Yield 3.4 g (22%, based on starting glycine): mp 146.5-147.5°C (dec) (lit. [2] mp 145°C, dec); [α]25D -66° (c 1.0, H2O) (lit. [2] -67°). Anal. Calcd for C8H15NO7: C, 40.51; H, 6.33; N, 5.91. Found: C, 40.18; H, 6.70; N, 5.58.
References:
Xing, Haoran;Mossine, Valeri V.;Yaylayan, Varoujan [Carbohydrate Research,2020,vol. 491,art. no. 107985] Location in patent:supporting information