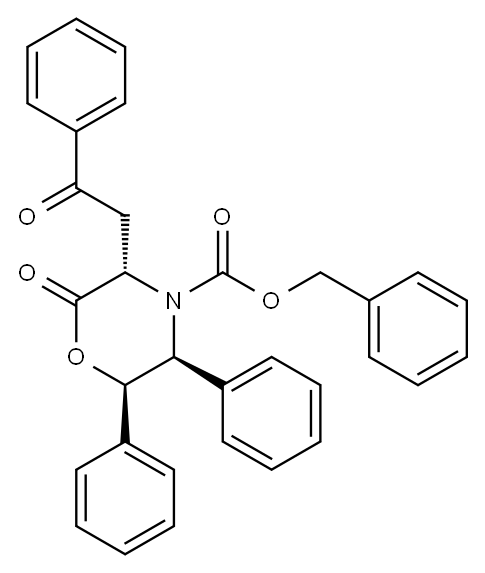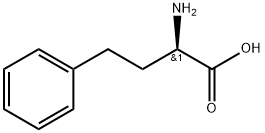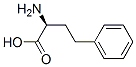
L-Homophe-OH synthesis
- Product Name:L-Homophe-OH
- CAS Number:943-73-7
- Molecular formula:C10H13NO2
- Molecular Weight:179.22
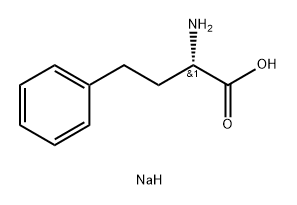
108960-95-8
0 suppliers
inquiry

943-73-7
483 suppliers
$5.00/1g
Yield:943-73-7 89.6%
Reaction Conditions:
with hydrogenchloride;sodium hydroxide in water;
Steps:
14 EXAMPLE 14
EXAMPLE 14 10.0 g of homophenylalanine sodium salt are dissolved in 60 ml of deionised water and the solution is added dropwise over 1 hour to 24 ml of 2N hydrochloric acid to form a white, faintly lustrous crystalline suspension. This suspension is adjusted with 1N sodium hydroxide solution to pH 4.0 and then stirred for 2 hours at room temperature and filtered. The filter product is washed with deionised water and dried at room temperature in a high vacuum, affording pure (+)-S-homophenylalanine with an angle of rotation of [α]D20 =+45.6° (1%, 1N hydrochloric acid). Melting point: 287°-290° C. Yield: 89.6%.
References:
US4785089,1988,A
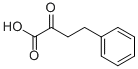
710-11-2
182 suppliers
$20.00/500mg

943-73-7
483 suppliers
$5.00/1g
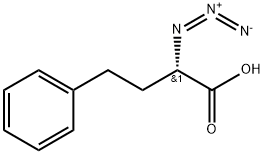
139040-47-4
0 suppliers
inquiry

943-73-7
483 suppliers
$5.00/1g
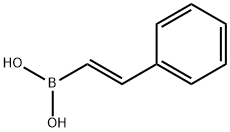
6783-05-7
105 suppliers
$24.00/1g

943-73-7
483 suppliers
$5.00/1g