Memantine HCl synthesis
- Product Name:Memantine HCl
- CAS Number:41100-52-1
- Molecular formula:C12H22ClN
- Molecular Weight:215.76
19982-07-1
222 suppliers
$14.00/250mg

41100-52-1
650 suppliers
$14.00/1g
Yield:41100-52-1 99.3%
Reaction Conditions:
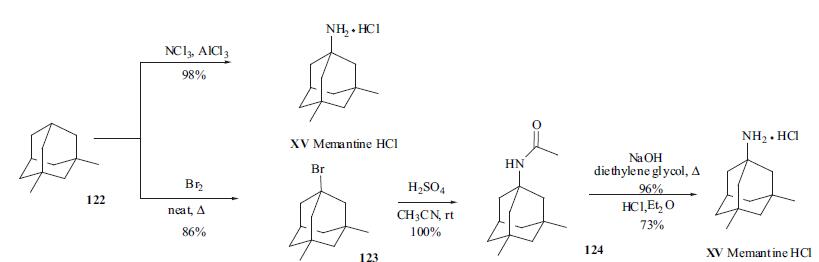
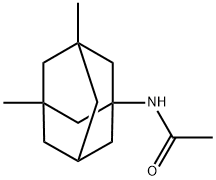
Stage #1: 1-acetamido-3,5-dimethyladamantanewith sodium hydroxide in diethylene glycol; for 6 h;Reflux;
Stage #2: with water in dichloromethane;diethylene glycol at 20; for 0.25 h;
Stage #3: with hydrogenchloride in ethyl acetate at 0 - 30; for 6 h;
Steps:
2
l-Acetamido-3,5-dimethyladamantane (44 gm) as obtained in example 1, sodium hydroxide and diethylene glycol are added, and heated to reflux. Then, the contents were maintained for 6 hours at reflux and water (1 175 ml) was added under stirring. The reaction mass was cooled to below 20 C and then added methylene chloride (750 ml), and stirred for 15 minutes at 20°C. The layers were separated and the organic layer was distilled off completely under vacuum at below 45°C to obtain a residue. The residue was dissolved in ethyl acetate (700 ml). Ethyl acetate hydrochloride (160 ml) was added to the reaction mass and stirred for 5 hours at 25 to 30°C. The reaction mass was cooled to 0 to 5°C and stirred for 1 hour at 0 to 5 °C. The separated solid was filtered and dried at 50°C for 2 hours to obtain 87 gm of memantine hydrochloride containing l-amino-3,5,7-trimethyladamantane hydrochloride and l-amino-3-methyladamantane hydrochloride impurity.Memantine hydrochloride: 99.3%;The combined contents of l-amino-3,5,7-trimethyladamantane hydrochloride and 1- amino-3-methyladamantane hydrochloride impurity: 0.5%.
References:
WO2011/125062,2011,A1 Location in patent:Page/Page column 8-9
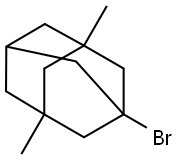
941-37-7
409 suppliers
$26.00/5g

41100-52-1
650 suppliers
$14.00/1g

6588-68-7
34 suppliers
inquiry

41100-52-1
650 suppliers
$14.00/1g
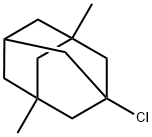
707-36-8
181 suppliers
$50.00/2g

41100-52-1
650 suppliers
$14.00/1g