METCONAZOLE synthesis
- Product Name:METCONAZOLE
- CAS Number:125116-23-6
- Molecular formula:C17H22ClN3O
- Molecular Weight:319.83
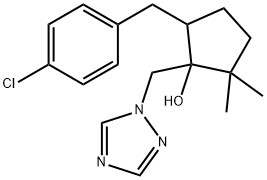
![Cyclopentanone, 5-[(4-chlorophenyl)methyl]-2,2-dimethyl-](/CAS/20210111/GIF/115851-28-0.gif)
115851-28-0
12 suppliers
inquiry
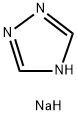
41253-21-8
276 suppliers
$5.00/5g
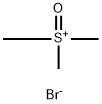
25596-24-1
197 suppliers
$6.00/10g

125116-23-6
120 suppliers
$85.00/100mg
Yield:125116-23-6 85%
Reaction Conditions:
with sodium hydroxide in 1-methyl-pyrrolidin-2-one at 110; for 5 h;Flow reactor;Inert atmosphere;Reagent/catalyst;Solvent;Temperature;
Steps:
1-2 A method for synthesizing metconazole, specifically:
In a 500 ml reaction flask equipped with a stirrer, a thermometer, a gas introduction tube and a distillation device,Add 50 grams (about 0.2 mole) of 2,2-dimethyl-5-(4-chlorobenzyl)cyclopentanone with a purity of 95% (the same below) by mass percentage,18.5 grams of sodium 1,2,4-triazole (about 0.2 mole), 5 grams (0.125 mole) of solid sodium hydroxide and 120 ml of N-methylpyrrolidone, stir and heat to 110°C; Start nitrogen bubbling (flow rate is about 30 liters/min), while bubbling nitrogen, add 38 grams (about 0.22 moles) of trimethylsulfoxonium bromide in batches for 4 hours.After the addition, bubbling was continued for 1 hour. HPLC analyzed that the molar percentage content of 2,2-dimethyl-5-(4-chlorobenzyl)cyclopentanone was less than 1%. The nitrogen bubbling was stopped and about 10 ml was evaporated. liquid. Concentrate under reduced pressure, add water and dichloroethane, extract the layers, concentrate, add methanol to recrystallize, and dry to obtain 54.5 g of metconazole as a white solid. The yield is 85%.
References:
Shanghai Heteng Fine Chemical Co., Ltd.;Shi Guancheng;Hu Haojie;Meng Haicheng CN111718304, 2020, A Location in patent:Paragraph 0031-0034
288-88-0
779 suppliers
$10.00/5g
![Cyclopentanone, 5-[(4-chlorophenyl)methyl]-2,2-dimethyl-](/CAS/20210111/GIF/115851-28-0.gif)
115851-28-0
12 suppliers
inquiry
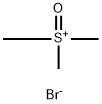
25596-24-1
197 suppliers
$6.00/10g

125116-23-6
120 suppliers
$85.00/100mg

288-88-0
779 suppliers
$10.00/5g
![Cyclopentanone, 5-[(4-chlorophenyl)methyl]-2,2-dimethyl-](/CAS/20210111/GIF/115851-28-0.gif)
115851-28-0
12 suppliers
inquiry

125116-23-6
120 suppliers
$85.00/100mg
![Cyclopentanone, 5-[(4-chlorophenyl)methyl]-2,2-dimethyl-](/CAS/20210111/GIF/115851-28-0.gif)
115851-28-0
12 suppliers
inquiry

41253-21-8
276 suppliers
$5.00/5g

125116-23-6
120 suppliers
$85.00/100mg

288-88-0
779 suppliers
$10.00/5g
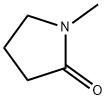
872-50-4
861 suppliers
$10.00/100ML
![Cyclopentanone, 5-[(4-chlorophenyl)methyl]-2,2-dimethyl-](/CAS/20210111/GIF/115851-28-0.gif)
115851-28-0
12 suppliers
inquiry
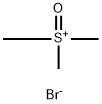
25596-24-1
197 suppliers
$6.00/10g

125116-23-6
120 suppliers
$85.00/100mg